1 Introduction
Generally, there are large number of people suffer from cognitive impairment, including impairment of learning, memory and attention.Citation1 However, the exact mechanisms for cognitive dysfunction have remained unclear, oxidative stress and neuroinflammation may be in part, responsible for memory and learning impairment.Citation2 In particular, the elderly people are most susceptible to cognitive impairment that may result from infection,Citation3 in addition, the process of aging itself is associated with neuroinflammation involving production of proinflammtory cytokines and microglial activation.Citation4 Also, neuroinflammation occurs in other conditions that may lead to pathological changes in the brain as postoperative dysfunction, ischemic stroke, Alzheimer disease (AD), multiple sclerosis or Parkinson disease.Citation5 In some animal models, exposure to bacterial endotoxins, or viral coat proteins, will stimulate immune system resulting in memory and learning deficits.Citation6
Lipopolysaccharide (LPS) is a component obtained from the outer cell membrane of gram-negative bacteria.Citation7 It is a potent endotoxin that inducing inflammation in experimental studies, and it highly resist the degeneration by enzymes and hence it provides an inflammatory stimulus that can persist for long time.Citation8 It can promote the production of proinflammatory cytokines with subsequent production of free radicals and oxidative stress.Citation9
Lactoferrin (LF) is a non-heme iron-binding glycoprotein of transferrin family.Citation10 It is present in milk, tears, saliva, and other external secretions, and in the secondary granules of neutrophils.Citation11 Normally, low concentrations of LF are present in the blood.Citation12 LF is a multifunctional protein that is involved in a large number of physiological functions including anti-inflammatory, anti-stress, anti-nociception, antioxidant, cell proliferative, and immunoregulatory properties,Citation13 regulation of iron absorption,Citation10 anticarcinogenic, and antimicrobial activity.Citation14 In addition, LF is produced in the brain, its levels in aging and neurodegenerative disorders are found to be increased in central nervous system, suggesting that it may have endogenous role in neuroprotection.Citation15 Dietary LF relatively resist degradation in gastrointestinal tract (GIT) and is actively transported into the brain.Citation16
However, to our knowledge, there were no published studies to date the effect of LF on memory impairment in adult rats. So, in the present study, we examined possible protective effect of LF on LPS-induced memory impairment in albino rats. Also, possible mechanisms would be discussed.
2 Materials and methods
2.1 Animals
Thirty male albino rats of local strain, aged 12–16 weeks old, weighing 200–240 g. They were obtained from the animal house of Tanta University of Medical Sciences. The animals were housed in clean cages, five rats per each cage, and had free access to food and water. They are maintained at suitable temperature (22 ± 2 °C room temperature) under controlled 12–12 h light dark cycle. This study was conducted in accordance with the guidelines for the animal experimental protocols of Tanta Faculty of Medicine.
2.2 Drugs and chemicals
LPS was dissolved in saline and injected in rats. It was purchased from Sigma-Aldrich chemical Company (Co.), LF was purchased from Meivo International Pharmaceutical Industries Co., in the form of sachet freshly prepared in distilled water.
2.3 Experimental protocols
Animals were divided into three groups (n = 10 per group).
| (1) | The normal control group: the animals received saline (1ml/kg/day) intraperitoneal (i.p.), as a vehicle, for 12 weeks. | ||||
| (2) | LPS-group: the animals received saline (1ml/kg/day) intraperitoneal (i.p.) for 11 weeks. From the start of the 12th week, the animals received LPS (1mg/kg/day i.p.)Citation17 for one week, and 2 h prior to passive avoidance (PA) test. | ||||
| (3) | LF-treated group: the animals received LF (500 mg/kg/day)Citation18 orally via intragastric tube for 12 weeks. From the start of the first week, the animals received LPS (1mg/kg/day i.p.) for one week, and 2 h prior to passive PA test. LPS was given 30 min after LF. | ||||
2.4 Passive avoidance (PA) test
At the end of the 12th week of experiment, PA test was done as following:
Light-dark apparatus was used to evaluate the emotional memory depending on fear-conditioning learning. The animals of all studied groups learned to avoid a place that associated with repulsive stimulus. The learning index in this experiment would be the reduction of latency. The PA apparatus consisted of two compartments. One was illuminated and the other was dark. The two compartments were separated by a door.
A training trial was done.Citation19 A pre-training trial was done on the first day of training, in that trial, rats were placed individually into the light compartment. The door between the two compartments was opened after 30 sec and the animal was able to move freely into the dark compartment.
60 min after the pre-training trial, a training trial was done. Rats were again placed in the light compartment. After 30 s, the door between the two compartments was opened. When the animal entered the dark compartment completely, the door was closed and 3–5 electric shock (0.5 mA) was applied to the floor of the compartment. The time taken to enter the dark compartment was estimated as the training latency. If the animal failed to enter the dark compartment within 300 sec, it was excluded from the experiment. After the training trial, the animals were removed from the dark compartment and returned back to their cages.
24-h after the training trial, a retention trial was done. Evaluation of recall of this inhibitory stimulus was done by placing the animals in the light compartment and recording their latency to enter the dark compartment. No electric shock was applied in this trial. If the animal did not enter the dark compartment within 300 sec, it was returned to its cage, and a latency of 300 sec was recorded. So, the latency is a measure for retention performance of the PA response.
2.5 Biochemical measurements
At the end of experiments, the animals were sacrificed by cervical decapitation. The brains were collected, they were homogenized followed by centrifugation. The supernatants were stored at −80 °C until measurement of the following: malondialdehyde (MDA) was measured spectrophotometerically according to the method of Csallany et al.,Citation20 which resulting in formation of a red compound. The maximum absorbance of this compound was measured at 532 nm, reduced glutathione (GSH) was measured spectrophotometerically according to the method described by Hissin and HilfCitation21 according to the instructions of the assay kit. Also, tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), nuclear factor kabba-B (NF-kB) and brain derived neurotrophic factor (BDNF) were determined by were determined using commercial ELISA kits according to the instructions of the manufacturer. Finally, acetylcholinestrase activity was estimated colorimetrically according to the method of Ellman et al.Citation22 and expressed as percentage of control. The protein content in the supernatant was measured according to the method of Bradford.Citation23
3 Statistical analysis
The data were shown as the mean ± standard deviation. Data from the study were analyzed using one–way ANOVA. All the analyses were performed using SPSS for windows (Version 21.0).
4 Results
4.1 Effect of LF on first day latency and retention latency of PA test in albino rats
As shown in , as regard the first day latency, there was no significant differences between all studied groups (P > 0.05). However, retention latency significantly reduced in LPS-group as compared to the normal control group. LF treatment for 12 weeks significantly increased retention latency as compared to the LPS-group. It was observed the retention latency returned back to the normal control value by LF treatment.
Table 1 Effect of LF on first day latency and retention latency of PA test in albino rats.
4.2 Effect of LF on brain levels of MDA and GSH
Compared to the control rats, LPS injection significantly increased MDA level in the brain. Administration of LF, significantly decreased MDA level as compared to the LPS-group. The levels of MDA returned back to the control values after LF treatment (A).
Fig. 1 Effect of LF on brain levels of MDA and GSH in albino rats. Data are given as mean ± SD. aP < 0.05 vs control group, bP < 0.05 vs LPS-group.
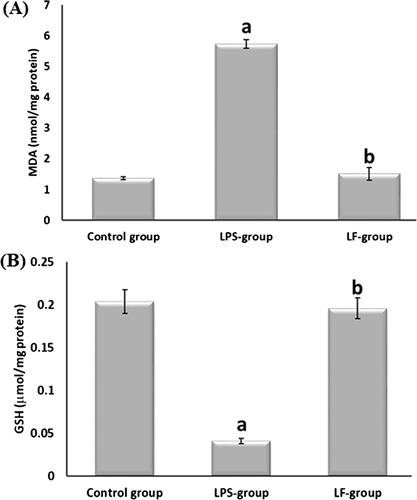
As regard GSH, there was significant decrease in GSH level in LPS-group as compared to the normal control group. While, LF treatment significantly increased GSH levels as compared to LPS-group. Its levels returned back to the normal control values by LF treatment (B).
4.3 Effect of LF on brain levels of TNF-α, IL-1β, and NF-κB in albino rats
These were presented in , the levels of TNF-α, IL-1β, and NF-κB significantly increased with LPS injection as compared to the normal control rats. While, LF treatment, showed significant reduction in TNF-α, IL-1β, and NF-κB levels compared to the LPS-group. But, their levels were still significantly higher if compared to the normal control group.
Table 2 Effect of LF on brain levels of TNF-α, IL-1β, and NF-κB, in albino rats.
4.4 Effect of LF on brain levels of BDNF and acetylcholinestrase activity in albino rats
(A) showed that BDNF significantly decreased after LPS injection as compared to the control group. LF administration significantly increased BDNF levels compared to the LPS-group. It was observed that after LF treatment, BDNF returned back to the control values.
Fig. 2 Effect of LF on brain levels of BDNF and acetylcholinestrase activity in albino rats. Data are given as mean ± SD. aP < 0.05 vs control group, bP < 0.05 vs LPS-group.
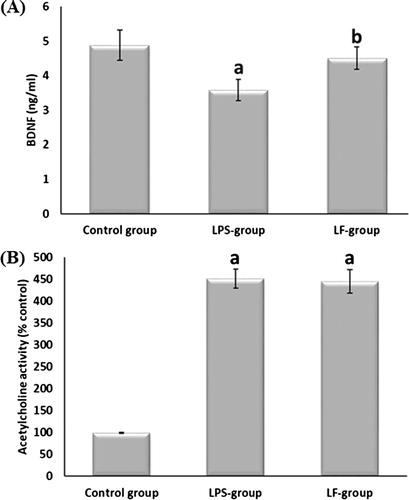
As shown in (B), LPS injection significantly increased levels of acetylcholinestrase activity compared to the control group. The rats treated with LF showed insignificant change in the levels of acetylcholinestrase activity compared to the LPS-group, but these levels significantly increased if compared to the normal control group.
5 Discussion
In the present study, LF improved memory impairment induced by i.p. administration of LPS in the adult rats. The neuroprotective effect of LF on memory impairment may be related to its antioxidant, anti-inflammatory effects, inhibiting the increase of NF-κB with subsequent inhibition of apoptotic pathway as well as increasing BDNF, but it had no effect on acetylcholinestrase activity in the brain.
The present study was the first one that was done to test the neuroprotective effect of LF on adult brain in rats. All previous studies were used to demonstrate the effect of LF on brains of immature or newly born rats.
PA test was used to detect the memory impairment caused by LPS injection. The results of the present study showed that no significant differences between all the studied groups during the first day latency, but there was a significant decrease in the time of latency to enter the dark compartment in LPS group as compared to the normal control group. These results were confirmed with previous researches reporting that LPS injection induced memory retention impairment of PA.Citation6
The results of our study showed that administration of LF for 12 weeks, improved the memory impairment in rats as proved by increasing the retention latency of PA as compared to LPS group. In accordance to these results, it was reported that the bovine LF administration during postnatal development of rats, improved PA test.Citation18
The exact mechanism by which LF improved LPS-induced memory impairment in rats could not be elucidated. But, it could be explained by its direct action on the brain via antioxidant, anti-inflammatory effects, and increasing BDNF as evidenced in the results of the present study. Another possible mechanism could explain the improvement of the memory impairment might be due to transferrin-mediated iron transport of LF in the brain, it could be evidenced by that iron deficiency during early life led to the subsequent impairment of both PA and active avoidance learning in rats.Citation24 Also, it was suggested that LF treatment promoted the growth of beneficial bacteria called bifidobacteria in the gut of the treated animals, it was observed that bifidobacteria might exert antidepressant or anxiolytic effects in both human and animal models.Citation25
The brain is highly susceptible to oxidative damage that has induced by reactive oxygen species (ROS), as it produces large amounts of ROS due to its very high blood perfusion, high aerobic metabolism and its poor antioxidant defense mechanism.Citation26 Therefore, oxidative stress of the brain plays an important role in cognitive impairment and neuronal injury.Citation27 Previous studies have demonstrated that i.p. injection of LPS caused significant changes in oxidative stress markers in the brain of mice.Citation28
In the results of the present work, there was significant increase of MDA levels with concomitant significant decrease of GSH levels in the brain in LPS group as compared to the normal control group. Also, our results showed that the abnormal changes in MDA and GSH levels were prevented by LF treatment, suggesting that the neuroprotective of LF might be explained by its antioxidant effects. These results were in accordance with previous researches demonstrating that LF has ROS-scavenging properties.Citation29
Previous studies have reported that LPS caused activation of microglia with subsequent induction of pro-inflammatory cytokines as TNF-α and IL-1β via activation of NF-κB pathway in the mouse brain.Citation30 In fact, activation of NF-κB leads to pathological inflammatory processes in the glial cells, hence, the transcription factor NF-κB could be blocked or reduced by anti-inflammatory compounds.Citation31
In the present study, i.p. injection of LPS significantly increase TNF-α and IL-1β levels in rat brain. While, LF treatment for 12 weeks significantly inhibited LPS-induced production of pro-inflammatory cytokines including TNF-α and IL-1β with concomitant reduction of NF-κB in the brain with subsequent blunting of neuronal apoptosis. Thus, LF had the ability to block the activation of NF-κB, suggesting that the neuroprotective effect of LF could be explained by its anti-neuroinflammatory and anti-apoptotic effects.
BDNF is one of a family of neurotrophic growth factors that widely expressed in the brain tissue.Citation32 The reduced levels of BDNF has been implicated in pathophysiology of various CNS diseases, BDNF regulates neuronal transmission, neuronal plasticity as well as memory and learning.Citation33
The results of the present study demonstrated that i.p. injection of LPS significantly reduced BDNF, that was reversed by LF treatment. It is proved that activation of BDNF leads to phosphorylation and nuclear translocation of cyclic adenosine monophosphate responsive element binding protein, with subsequent gene transcription that plays an important role in enhancing memory and learning processes.Citation34 Thus, we could postulate that the increase in BDNF by LF treatment may be one of the underlying mechanisms to explain how LF improved memory impairment in rats.
Finally, the present study showed that LPS injection significantly increased the acetylcholinestrase activity in the brain tissue that could not be improved by LF treatment, suggesting that LF did not affect acetylcholinestase activity.
6 Conclusion
As proved in the present work, LF could alleviate memory impairment induced by LPS. Its neuroprotective mechanism might be explained by antioxidant, anti-neuroinflammatory effects, its ability to inhibit NF-κB with subsequent anti-apoptotic effect, as well as increasing BDNF levels. So further studies should be done to investigate the neuroprotective effect of LF and other possible mechanisms for its action in human brain, and take in consideration for LF treatment that can be used for prophylaxis as well as treatment of neurodegenerative diseases resulting from inflammatory or oxidative damage.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
References
- S.A.EshkoorT.A.HamidC.Y.MunC.K.NgMild cognitive impairment and its management in older peopleClin Interv Aging102015687693
- H.CastelA.DenouelM.LangeM.C.TononM.DuboisF.JolyBiomarkers associated with cognitive impairment in treated cancer patients: potential predisposition and risk factorsFront Pharmacol2182017138
- A.A.SimenK.A.BordnerM.P.MartinL.A.MoyL.C.BarryCognitive dysfunction with aging and the role of inflammationTher Adv Chronic Dis232011175195
- S.Di BenedettoL.MullerE.WengeRS.DuzelG.PawelecContribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventionsNeurosci Biobehav Rev752017114128
- M.BergerV.PonnusamyN.GreeneThe Effect of propofol vs. isoflurane anesthesia on postoperative changes in cerebrospinal fluid cytokine levels: results from a randomized trialFront Immunol820171528
- A.AnaeigoudariM.N.ShafeiM.SoukhtanlooLipopolysaccharide-induced memory impairment in rats is preventable using 7-nitroindazoleArq Neuropsiquiatr7392015784790
- X.WangP.J.QuinNEndotoxins: lipopolysaccharides of gram-negative bacteriaSubcell Biochem532010325
- H.ZhouM.ChenG.ZhangR.D.YeSuppression of lipopolysaccharide-induced inflammatory response by fragments from serum amyloidA J Immunol1993201711051112
- W.LiS.YangS.O.KimG.ReidJ.R.ChallisA.D.BockingLipopolysaccharide-induced profiles of cytokine, chemokine, and growth factors produced by human decidual cells are altered by lactobacillus rhamnosus GR-1Supernatant Reprod Sci2172014939947
- L.RosaA.CutoneM.S.LepantoR.PaesanoP.ValentiLactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasisInt J Mol Sci1892017pii: E1985
- T.Siqueiros-CendonS.Arevalo-GallegosB.F.Iglesias-FigueroaI.A.Garcia MontoyaJ.Salazar-MartinezQ.Rascon-CruzImmunomodulatory effects of lactoferrinActa Pharmacol Sin3552014557566
- N.AnandR.K.KanwarM.L.DubeyEffect of lactoferrin protein on red blood cells and macrophages: mechanism of parasite-host interactionDrug Des Devel Ther9201538213835
- N.BruniM.T.CapucchioE.BiasibettiAntimicrobial activity of lactoferrin-related peptides and applications in human and veterinary medicineMolecules2162016pii: E752
- D.LegrandLactoferrin, a key molecule in immune and inflammatory processesBiochem Cell Biol9032012252268
- J.WangM.BiH.LiuN.SongJ.XieThe protective effect of lactoferrin on ventral mesencephalon neurons against MPP+ is not connected with its iron binding abilitySci Rep5201510729
- Y.Van de LooijV.GinetA.ChatagnerLactoferrin during lactation protects the immature hypoxic-ischemic rat brainAnn Clin Transl Neurol1122014955967
- A.AbareshiA.AnaeigoudariF.NorouziLipopolysaccharide-induced spatial memory and synaptic plasticity impairment is preventable by captoprilAdv Med201620167676512
- J.ShumakeD.W.BarrettM.A.LaneA.J.WittkeBehavioral effects of bovine lactoferrin administration during postnatal development of ratsBiometals27201410391055
- S.MonleonA.UrquizaC.Vinader-CaerolsA.ParraEffects of oxotremorine and physostigmine on the inhibitory avoidance impairment produced by amitriptyline in male and female miceBehav Brain Res20522009367371
- A.S.CsallanyM.D.GuanJ.D.ManwaringP.B.AddisFree malondialdehyde determination in tissues by high performance liquid chromatographyAnal Biochem14221984277283
- P.J.HissinR.HilfA fluorometric method for determination of oxidized and reduced glutathione in tissuesAnal Biochem741976214226
- G.L.EllmanK.D.CourtneyV.AndresJr.R.M.FeatherstoneA new and rapid colorimetric determination of acetylcholinesterase activityBiochem Pharmacol719618895
- M.M.BradfordA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnal Biochem721976248254
- E.C.RadlowskiR.W.JohnsonPerinatal iron deficiency and neurocognitive developmentFront Hum Neurosci72013585
- J.F.CryanT.G.DinanMind-altering microorganisms: the impact of the gut microbiota on brain and behaviorNat Rev Neurosci132012701712
- G.H.KimJ.E.KimS.J.RhieS.YoonThe Role of oxidative stress in neurodegenerative diseasesExp Neurobiol2442015325340
- A.H.BhatK.B.DarS.AneesOxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insightBiomed Pharmacother742015101110
- X.Y.ZhangJ.B.CaoL.M.ZhangY.F.LiW.D.MiDeferoxamine attenuates lipopolysaccharide-induced neuroinflammation and memory impairment in miceJ Neuroinflammation12201520
- H.BurrowR.K.KanwarJ.R.KanwarAntioxidant enzyme activities of iron-saturated bovine lactoferrin (Fe-bLf) in human gut epithelial cells under oxidative stressMed Chem732011224230
- L.QinJ.HeR.N.HanesO.PluzarevJ.S.HongF.T.CrewsIncreased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatmentJ Neuroinflammation5200810
- R.HaenoldF.WeihK.H.HerrmannNF-κB controls axonal regeneration and degeneration through cell-specific balance of RelA and p50 in the adult CNSJ Cell Sci127Pt 14201430523065
- S.S.JiaoL.L.ShenC.ZhuBrain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer's diseaseTransl Psychiatry6102016e907
- S.BathinaU.N.DasBrain-derived neurotrophic factor and its clinical implicationsArch Med Sci116201511641178
- E.RosaM.FahnestockCREB expression mediates amyloid β-induced basal BDNF downregulationNeurobiol Aging368201524062413
