Abstract
Background
Chronic kidney disease (CKD), has become a public health concern as it has been reported to cause adverse outcomes such as kidney failure and premature death. This cross sectional study compared the Kidney Disease: Improving Global Outcomes (KDIGO) and Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines in assessing the prevalence of CKD in Type 2 diabetes Mellitus (T2DM) patients.
Methods
We consecutively sampled a cross-section of 202 T2DM patients from the Ho municipality in the Volta region (Ghana). Structured pre-tested questionnaires were administered to obtain information on gender, age, body mass index (BMI), systolic and diastolic blood pressure, medication used, duration on medication, and duration of diabetes. Serum creatinine and urine protein were estimated using standard protocols and CKD was classified according to KDIGO and KDOQI guidelines.
Results
The prevalence of CKD was 63.4% and 58.4% using the KDIGO and KDOQI guidelines respectively. The prevalence of mildly decreased renal function or worse (eGFR < 60/ml/min/1.73 m2) was 10.4% for KDIGO guideline and 7.9% for KDOQI guidelines with an excellent agreement between both definitions showing bias = −0.129, 95%CI = (−0.17 to −0.08) on Bland-Altman analysis. Participants older than 70 years were more likely to have CKD when KDIGO criteria was used (P = 0.018). The prevalence of albuminuria was 47.0% with 21.9% presenting with 1+ and 2+ grades.
Conclusion
KDIGO guideline estimates higher prevalence of CKD than KDOQI guidelines in the same study population. KDIGO guideline might help in early detection and proper classification of CKD which will illicit stage-specific treatment.
1 Background
Chronic kidney disease (CKD) is a global health concern with adverse outcomes. Many studies have reported that CKD affects between 5% and 15% of the adult population.Citation1–Citation4 Type 2 diabetes mellitus (T2DM) is the leading cause of CKD in the US with previous estimates suggesting close to 40% of patients with T2DM showing evidence of CKD.Citation1 Adverse outcomes associated with CKD include End-Stage Renal Disease (ESRD) and premature death.Citation1 Previous studies from Africa show a prevalence of CKD of 10.4% and proteinuria of 12.4%.Citation5 We have previously reported that the prevalence of CKD in T2DM patients was 30%Citation6 which was attributed to several other major risk factors such as obesity, hypertension, and use of anti-diabetic drugs.Citation7,Citation8
A uniform CKD staging and classification system was proposed by Kidney Disease Outcomes Quality Initiative (KDOQI) based on estimated glomerular filtration rate (eGFR).Citation9 The original classification ranged from Stage 1 CKD (defined as normal kidney function with other markers for kidney damage) to Stage 5 CKD (defined as kidney failure). The aim of this staging system was to provide clinicians with stage-specific action plans for treatment of CKD and associated comorbidities and complications. It also enhanced research and served as a framework for developing a public-health approach to CKD.Citation1
However, Kidney Disease: Improving Global Outcomes (KDIGO) recently updated the classification system for CKD based on emerging evidence developed by Go and his colleagues.Citation10 This new classification system is based on the cause of CKD, GFR category, and albuminuria category (CGA). The cause category is based on the presence or absence of systemic disease. The GFR categories acknowledge the need to subdivide Stage 3 CKD, classified as G3 under the new classification system, into G3a (eGFR 45–59) and G3b (eGFR 30–44). The albuminuria categories are based on the presence and severity of albuminuria. This new classification was developed to allow risk stratification based on progression of CKD, cardiovascular diseases and other complications.Citation3
Most studies on CKD use either the KDIGO or KDOQI guidelines to stage CKD.Citation10–Citation12 Although Bailey et al.,Citation1 revealed that KDIGO guidelines estimates CKD higher than KDOQI guidelines in the same study participants, same cannot be said in the African setting since there is limited data on such studies at the moment. Driven by the hypothesis that KDIGO estimates higher prevalence than KDOQI in the same study participants, we compared KDIGO and KDOQI guidelines in the assessment of CKD out-patient diabetes patients in the Ho Municipality (Ghana) so as to throw more light on the need for early identification and proper staging of CKD. This we postulated would subsequently lead to commencement of early treatment directed at slowing or preventing progression, and treatment of associated complications and comorbidities.
2 Methodology
2.1 Study setting
A non-randomized cross-sectional study was conducted at the outpatient diabetes unit of the Ho Municipal Hospital (HMH), from January to March 2017. A 150 bed capacity facility, located in the Volta region, HMH is the main referral center providing general and specialist health care services for over 265,046 inhabitants of the Ho Municipality and Adaklu-Anyigbe District in the Volta region of Ghana.
2.2 Study population
Eligible male and female participants (>35 years) were enrolled from the diabetes unit of the hospital. Patients with chronic conditions like HIV/AIDS, cancer, congestive cardiac failure, liver disease, respiratory failure were excluded. We also excluded patients with a baseline creatinine of >1.5 mg or with an eGFR of <60 mL/min, narcotic drug users and those with a history of hepatitis B and C.Citation6
2.3 Study design and data collection
We consecutively sampled 202 eligible participants for the study. T2DM was defined according to standard protocols.Citation15 Demographic, anthropometric, and clinical data (systolic and diastolic blood pressure, medication used, duration on medication, and duration of diabetes) were obtained with a questionnaire and from patient’s medical records.
2.4 Blood pressure measurement
A standard mercury sphygmomanometer and stethoscope (ACCOSON, England) was used to measure blood pressure in accordance with recommendations of the American Heart Association.Citation13
2.5 Anthropometry
Height (cm) and weight (kg) were measured with a wall-mounted ruler and a bathroom scale (Zhongshan Camry Electronic Co. Ltd, Guangdong, China) respectively. BMI was calculated by and categorized according to WHO criteria into normal weight (BMI 18.5–24.9), underweight (<18.5), overweight (25.0–29.9), obese (30.0–39.9).Citation14
2.6 Biochemical analysis
Serum creatinine was measured with an automated chemistry analyzer (SELECTRA PRO XS). Estimated GFR was calculated with the 4v-MDRD and the CKD-EPI equations.Citation17
Albuminuria was measured with a dipstick (URS-2T) and staged as ‘A1’ (albumin concentration < 30 mg/dl) and ‘A2’ (30–299 mg/dl). CKD was defined and staged based on the KDIGO and the KDOQI guidelines.Citation18,Citation19
2.7 Statistical analysis
Data was analyzed with Graphpad prism version 5.0 (GraphPad software, San Diego California USA, http://www.graphpad.com). Two-sample Student’s t test and chi-square or Fisher’s exact test, as appropriate, and one-way analysis of variance (ANOVA) were used to compare groups. A P-value ≤ 0.05 was considered statistically significant. Bland-Altmann analysis was done to test agreement between the two guidelines.
3 Results
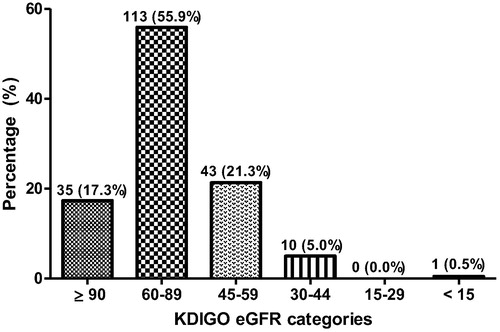
Classification of eGFR using KDIGO criteria as shown in reveals that most (55.9%) of the participants had eGFR of (60–89 mL/min/1.73 m2), while 21.3% also had eGFR of (45–59 mL/min/1.73 m2). None of the participants had eGFR 15–29 mL/min/1.73 m2.
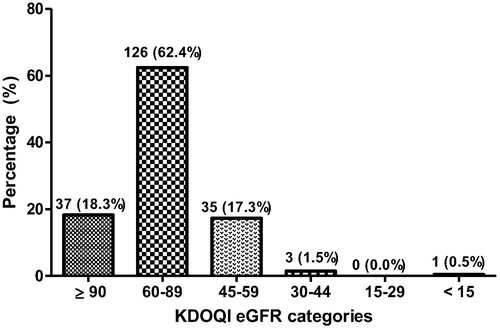
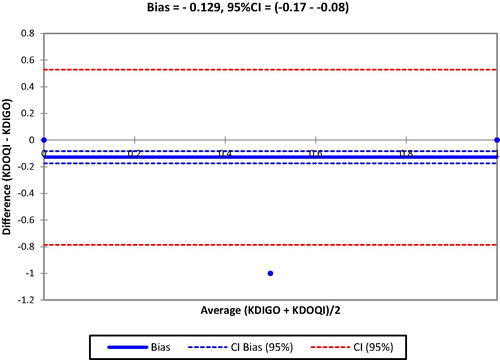
describes the classification of eGFR using KDOQI criteria. Most (62.4%) of the participants had eGFR of (60–89 mL/min/1.73 m2), while 18.3% also had eGFR ≥ 90 mL/min/1.73 m2. In the Bland-Altman analysis above, there was an excellent agreement between KDIGO and KDOQI definitions Bias = −0.129, 95%CI = (−0.17 to −0.08) (see ).
Fig. 3 Degree of agreement between KDIGO and KDOQI definitions in diagnosing chronic kidney disease.
shows the prevalence of CKD based on KDIGO and KDOQI criteria. Of the study participants, 128 (63.4%) had CKD based on the KDIGO criteria as compared to 118(58.4) for the KDOQI criteria. For the staging of CKD, 26.7% had stage 2 CKD while 21.3% had stage 3a CKD based on the KDIGO criteria. Only one patient had end stage CKD (stage 5) using both KDIGO and KDOQI criteria.
Table 1 Prevalence of CKD among participants.
describes the prevalence of albuminuria and estimated glomerular filtration rate (eGFR). As shown in the table, the prevalence of albuminuria was 47.0% with 21.9% presenting with 1+ and 2+ grades. KDIGO criteria diagnosed more patients with low eGFR (<60 mL/min/1.73 m2) (26.7%) compared with 19.3% as recorded by the KDIGO criteria.
Table 2 Prevalence of albuminuria, estimated glomerular filtration rate (eGFR).
describes the prevalence of CKD based on albuminuria staging using KDIGO criteria. For participants with stage 2 CKD and eGFR 60–89 mL/min/1.73 m2, the prevalence of albuminuria was 26.7% with 10.9% presenting with severe forms (30–299 mg/dl). Also the prevalence of albuminuria among patients with stage 3a CKD (eGFR 30–44 mL/min/1.73 m2) was 8.9% with 4% showing severe albuminuria (30–299 mg/dl). One (1) patient had stage 5 CKD with eGFR < 15 and presented with severe albuminuria (30–299 mg/dl) based on the KDIGO criteria.
Table 3 Prevalence of CKD among diabetic patients based on Albuminuria staging using KDIGO criteria.
shows the prevalence of CKD based on albuminuria staging using KDOQI criteria. For participants with stage 1 CKD and eGFR ≥ 90 mL/min/1.73 m2, the prevalence of albuminuria was 11.9% with 6.4% presenting with severe forms (30–299 mg/dl). 10.9% of the participants had stage 2 CKD and severe albuminuria (30–299 mg/dl). Also the prevalence of albuminuria among patients with stage 3a CKD (eGFR 30–44 mL/min/1.73 m2) was 6.9% with 3.5% showing mild albuminuria (<30 mg/dl). Only one (1) patient had stage 5 CKD with eGFR < 15 and presented with severe albuminuria (30–299 mg/dl).
Table 4 Prevalence of CKD among diabetic patients based on Albuminuria staging using KDOQI criteria.
shows demographic characterizations of diabetic patients as determinants of CKD. This depicts that participants older than 70 years were more likely to have CKD when KDIGO criteria was used (P = 0.018).
Table 5 Demographic characterizations of diabetic patients as determinants of CKD.
4 Discussion
In this study we compared two chronic kidney disease classification systems (KDIGO and KDOQI) regarding their ability to properly define and accurately estimate the prevalence of CKD among T2DM patients in the Ho municipality in Ghana. We found that using KDIGO guideline estimated higher CKD prevalence (63.4%) than the KDOQI guideline which estimated 58.4%. This is because KDIGO classified the distribution of albuminuria by severity within the categories of eGFR- providing insights into the distribution of risk categories for progression to ESRD and mortalityCitation1 as KDIGO and KDOQI had similar prevalence rate for stages 1 and 2. There was an excellent agreement between KDIGO and KDOQI definitions on Bland-Altman analysis which suggests that, KDIGO may help in early identification and proper categorization of CKD which may allow for timely and specific interventional therapy to prevent complications and reduce mortality.Citation1,Citation8,Citation9 In a similar study conducted among T2DM patients by Janmohamed et al.Citation11 in Tanzania, they reported a CKD prevalence of 83.7% which is higher than the 63.4% recorded when the KDIGO guideline was used in our study. However, Mpondo et al.Citation15 later stated that the high prevalence of CKD observed in Janmohamed’s study could be attributed to the schistosomiasis endemicity at the study site (along the shores of Lake Victoria) which had been reported to cause a range of renal diseases. The possibility of schistosomiasis as a confounder may have accounted for the lower CKD prevalence observed in our study. A recent report by Plantinga et al.Citation16 using a similar staging of CKD reported a slightly lower prevalence of CKD than that reported in this study. The Plantinga et al.Citation16 report showed a lower mean age, and included T1DM patients and those diagnosed with T2DM age < 30 years. A previous report by Coresh et al.Citation4 observed that the prevalence of CKD increased with age, suggesting that the criteria used for this study may explain some of the differences observed. This is because the kidney declines in functions with ageing but CKD does not progress beyond the moderate stage unless other conditions such as diabetic kidney disease develops.Citation17 Nearly 27% of our study participants had an eGFR < 60 mL/min/1.73 m2 using the KDIGO guideline as compared to 19% by the KDOQI guideline. This is consistent with a study conducted by Bailey et al.Citation1 An eGFR < 60 mL/min/1.73 m2 suggest loss of half or more of adult kidney function and is a baleful finding in a setting where there is limited options for advanced kidney disease patients. A low eGFR might also have implications for drug therapy for diabetes mellitus and if undetected could lead to complications and poor clinical outcome. We also observed that, CKD increased with age as majority of participants aged 60 years and above were captured by both guidelines which is consistent with a systematic review conducted by RitzCitation17 with data from Europe, America and Africa. This study also identified a fewer number of the participants with mild to severe CKD (stages 3a-4) as majority (26.3 for KDIGO and 18.8 for KDOQI) were diagnosed with mild CKD and only about 1% with End Stage Renal Disease (ESRD). Even though the duration of the diabetes might be significant to cause severe kidney damage, almost all the participants visited the diabetic clinic for regular checkups which could account for our findings.
Our study comes with numerous limitations. First, our study was a single-centre study. Thus, it is likely that the prevalence rates estimated in other urban and rural towns in the Volta region and across Ghana as a whole may vary. Second, the study is also limited by the small sample size and use of the single measurement of serum creatinine (whereas to truly fulfill definitions of CKD, two measurements at least 3 months apart are needed). In addition, the creatinine measured was not standardized by IDMS. Fourth, the study failed to compare the eGFR to the gold standard that is inulin clearance. Finally, based on the data collected, the cause of CKD was not captured, even though our participants were T2DM patients, hence we could not categorize CKD according to the cause category of the new KDIGO classification.
However the consecutive sampling and completeness of data collection strengthens our findings. Further studies should be conducted to give more insight into diagnosing CKD using either of the two guidelines as there is scanty data in sub-Saharan Africa.
5 Conclusion
KDIGO guideline estimated higher prevalence of CKD than KDOQI guidelines in a population of T2DM patients in an urban community. KDIGO guideline may help identify the development of CKD early enough to curtail associated complications and mortality. KDIGO guideline might help in early detection and proper classification of CKD which will illicit stage-specific treatment.
Acknowledgement
We acknowledge the immense efforts of the staff of the laboratory department of the Ho Municipal Hospital as well as patients of the diabetic clinic in making this work a success.
Competing interests
The authors declare that they have no conflict of interest in this manuscript.
Authors’ contributions
RKDE, SA, WT and SAS conceived of the study and participated in its design and coordination. SA, RKDE, HA and WT were involved in the recruitment of participants, data collection and analysis. RKDE, SAS, LAF, RM and FAB drafted the manuscript. RKDE, SAS, LAF and BA provided analytic and statistical support. All authors read and approved the final manuscript.
Ethical considerations
The study was approved by the University of Cape Coast institutional review board (UCC/IRB) and the ethics committee of HMH. Written informed consent was obtained from all participants prior to data collection.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 1 August 2018
References
- R.A.BaileyY.WangV.ZhuM.F.RupnowChronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) stagingBMC Res Notes72014415
- S.J.ChadbanE.M.BrigantiP.G.KerrPrevalence of kidney damage in Australian adults: The AusDiab kidney studyJ Am Soc Nephrol14suppl 22003S131S138
- N.ChenW.WangY.HuangCommunity-based study on CKD subjects and the associated risk factorsNephrol Dial Transplant24200921172123
- J.CoreshE.SelvinL.A.StevensPrevalence of chronic kidney disease in the United StatesJama298200720382047
- M.O.AfolabiE.Abioye-KuteyiF.A.ArogundadeI.S.BelloPrevalence of chronic kidney disease in a Nigerian family practice populationSouth African Family Practice512009132137
- R.K.EphraimS.BiekpeS.A.SakyiP.AdobaH.AgbodjakeyE.O.AntohPrevalence of chronic kidney disease among the high risk population in South-Western Ghana; a cross sectional studyCan J Kidney Health Dis2201540
- A.M.HungC.L.RoumieR.A.GreevyComparative effectiveness of incident oral antidiabetic drugs on kidney functionKidney Int812012698706
- A.S.LeveyL.A.StevensEstimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictionsAm J Kidney Dis552010622627
- A.S.LeveyJ.CoreshK.BoltonK/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratificationAm J Kidney Dis392 SUPPL. 12002
- A.GoG.ChertowD.FanChronic kidney disease and the risks of death, cardiovascular events, and hospitalizationJ Vasc Surg412005177
- M.N.JanmohamedS.E.KalluvyaA.MuellerPrevalence of chronic kidney disease in diabetic adult out-patients in TanzaniaBMC Nephrol142013183
- C.OsafoM.Mate-KoleK.AfframD.AduPrevalence of chronic kidney disease in hypertensive patients in GhanaRenal Failure332011388392
- T.G.PickeringJ.E.HallL.AppelResponse to recommendations for blood pressure measurement in human and experimental animals; part 1: blood pressure measurement in humans and miscuffing: a problem with new guidelines: addendumHypertension4812006e5e6
- Status WP. The use and interpretation of anthropometry. Geneva CH. In: WHO 1995, technical report 854; 1995.
- B.C.MpondoE.NeilsonA.ErnestPrevalence of chronic kidney disease in diabetic adult out-patients in TanzaniaBMC Nephrol17201671
- L.C.PlantingaD.C.CrewsJ.CoreshPrevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetesClin J Am Soc NephrolCJN201007891109
- E.RitzS.R.OrthNephropathy in patients with type 2 diabetes mellitusN Engl J Med341199911271133
- http://kdigo.org/guidelines/ckd-evaluation-and-management/.
- http://www.kidney.org/professionals/KDOQI/news_ckd_classification.