Abstract
Objective
Non Hodgkin Lymphomas (NHL)s are a group of malignancies which affect the lymphatic system. A significant proportion of NHL patients experience either relapse or failure of treatment which is called refractory disease. Relapsed or refractory NHL usually have poor prognosis due to shortage of randomized trials comparing efficacy of different treatment protocols to define the optimal salvage chemotherapy regimen in these cases. In this study, we are trying to define the best salvage chemotherapy regimen with low toxicity and better quality of life for patients by comparing outcome of 2 salvage chemotherapy regimens GDP & DHAP.
Patients and methods
100 patients diagnosed as relapsed or refractory NHL were randomly assigned to receive either Gemcitabine, Dexamethasone and Cisplatin (GDP) or Dexamethasone, Cytarabine and Cysplatin (DHAP) for 4 to 6 cycles. Primary endpoints of the study were overall survival and progression free survival. Secondary endpoints were response to treatment, toxicity profile of each regimen, and quality of life assessment.
Results
The overall response rate was 70% in GDP group & 64% in DHAP group with no statistically significant difference between them (p-value 0.5). There was no significant difference between both groups regarding toxicity profile except in febrile neutropenia episodes which was much less in GDP group (p-value 0.04). Quality of life was better in GDP group than DHAP with significant difference (p-value < 0.05). There was no statistical significant difference between both groups regarding OS or PFS.
Conclusion
GDP is as effective as DHAP for relapsed or refractory lymphoma with less toxicity and better quality of life.
Keywords:
1 Introduction
Non Hodgkin Lymphomas (NHL)s are a group of malignancies which affect the lymphatic system, they result from uncontrolled monoclonal proliferation of B or T lymphocytes and natural killer (NK) cells.Citation1
Although aggressive NHLs are characterized by fast growth and short survival with increasing number of patients being cured by intensive chemotherapy, a significant proportion of NHL patients experience either relapse or failure of treatment which is called refractory disease.Citation2
Relapsed or refractory NHL usually have poor prognosis due to different biologic and gene expression markers of the disease, drug resistance and patient related factors which lead to difficulty in defining the optimal salvage chemotherapy regimen in these cases.Citation3
In developing countries with limited resources as Egypt, high dose chemotherapy followed by autologous stem cell transplant (ASCT) is not always an option of treatment in relapsed and refractory lymphomas due to low number of transplant centers across the country, long waiting lists and limited resources.
So, in this study we are trying to define the best salvage chemotherapy regimen with low toxicity and better quality of life to be offered to this group of patients with poor prognosis.
2 Patients and methods
This prospective randomized study was conducted at Clinical Hematology Unit, Internal Medicine Department and Medical oncology department, Faculty of Medicine, Assiut University, between January 2013 and December 2016. Eligibility criteria were defined as all patients more than 18 years of age with histologically proven B or T cell NHL which was refractory to primary treatment or relapsed after one chemotherapy regimen, with measurable lesion, normal liver and kidney functions and Eastern Cooperative Oncology Group (ECOG) performance status from zero to two. This study included one hundred patients who were randomly assigned to receive either Gemcitabine, Dexamethasone and Cisplatin (GDP) or Dexamethasone, Cytarabine and Cysplatin (DHAP) for 4 to 6 cycles.
Patients were randomized into 2 groups: Group (A) which consisted of 50 patients who received GDP in the form of Gemcitabine 1000 mg/m2 I.V on days 1&8, Dexamethasone 40 mg I.V on days 1–4 and Cisplatin 75 mg/m2 I.V on day 1 every 21 days for 4–6 cycles and Group(B) which consisted of 50 patients who received DHAP in the form of Dexamethasone 40 mg on days 1–4, Cytarabine 2 g/m2 on days 2&3 and Cisplatin 35 mg/m2 on days 1–3 every 21 days for 4–6 cycles. Ondansteron and dexamethasone were given as antiemetics before each cycle in both regimens. GDP was given as an outpatient regimen while DHAP was an inpatient regimen.
All patients in both groups were subjected to full history, complete physical examination, routine baseline laboratory investigations, excisional lymph node biopsy with immunohistochemistry to confirm B or T lineage and subtype of NHL, bone marrow aspirate and biopsy with immunophenotyping if indicated and multislice CT scans of chest and abdomen to measure the burden of the disease.
Complete reevaluation of the patients to assess response to treatment was done after 2nd, 4th and 6th cycle and response was determined using International Workshop NHL response criteria.Citation4 Toxicity to treatment was evaluated after the end of every 2 cycles of treatment according to the Common Toxicity Criteria of the National Cancer Institute.Citation5
Assessment of quality of life was done using the Functional Assessment of Cancer Therapy (FACT)Citation6 which considers a change of 10% or more compared to baseline quality of life a meaningful change and classified into improved, stable and worse.
A written informed consent was obtained from all patients and approval or research ethics committee of Assiut faculty of medicine was obtained before the study.
The primary endpoint of the study is to detect overall survival and progression free survival and the secondary endpoints are response rate to treatment, toxicity and quality of life assessment.
2.1 Statistical analysis
Data entry and data analysis were done using Statistical Package for Social Science (SPSS). Data were presented as number, percentage, mean, standard deviation, standard error, median and range. Chi-square test was used to compare quantitative variables between two groups. P-value considered statistically significant when P < 0.05. Survival curves were done using Kaplan-Meier Survival analysis and log rank test was used to compare between survival curves.
3 Results
This study included one hundred NHL patients. Fifty patients received GDP, they were 27 males (54%) & 23 females (46%) with median age of 40 years (24–65), while DHAP regimen was given to another 50 patients, 32 males (64%) & 18 females (36%) with age ranged from 22 to 69 years ().
Table 1 Patients characteristics.
In patients who received GDP constitutional symptoms were present in 30 patients (60%) and were absent in 20 patients (40%), while in patients received DHAP they were present in 32 patients (64%) and absent in 18 patients (36%).
Most of the patients included in the study have high IPI risk factor with 56% in GDP group and 44% in DHAP group. 33 patients (66%) were diagnosed as B-NHL in GDP group and 30 patients (60%) in DHAP group while 17 (34%) and 20(40%) were diagnosed as T-NHL in GDP and DHAP groups.
According to response to previous therapy, 14(28%) were refractory to treatment in both groups, 23 patients (46%) and 20 (40%) had a relapse within less than one year in GDP and DHAP groups and 13(26%) and 16(32%) had a relapse more than one year in GDP and DHAP groups respectively. None of the patients in both groups had received Rituximab as a part of primary therapy due to high cost.
Overall response to treatment was observed in 35 patients (70%) who received GDP and 32 patients (64%) who received DHAP. Partial response was observed in 40% and 36% of patients receiving GDP and DHAP while complete response was observed in 30% and 28% of patients in GDP and DHAP groups. However, there was no statistical significance difference between both groups regarding overall, partial or complete response with p-values 0.5, 0.6 and 0.7 respectively (). 5 patients (10%) progressed on treatment with GDP and 8 patients (16%) progressed on treatment with DHAP with no statistical difference.
Table 2 Overall response rate.
Regarding toxicity, there was no statistical difference between both groups except in episodes of febrile neutropenia which occurred in 36% and 60% of patients receiving GDP and DHAP with p-value 0.04 (). Red cell transfusions were needed in 15% of patients receiving GDP and 25% in patients receiving DHAP while platelet transfusions were needed in 12% and 15% of patients receiving GDP and DHAP. Only 2 patients (4%) receiving GDP had interrupted treatment while 10 patients (20%) receiving DHAP had interrupted treatments and all due to episodes of febrile neutropenia.
Table 3 Adverse effects of both regimens.
According to quality of life assessment, there was statistically significant difference between both groups where 20 patients (40%) and 8 patients (16%) were improved in GDP and DHAP groups, 19 patients (38%) and 16 (32%) were stable in GDP and DHAP while, 11 patients (22%) and 26(52%) got worse during treatment with GDP and DHAP with p-value <0.01 ().
Table 4 Quality of life assessment.
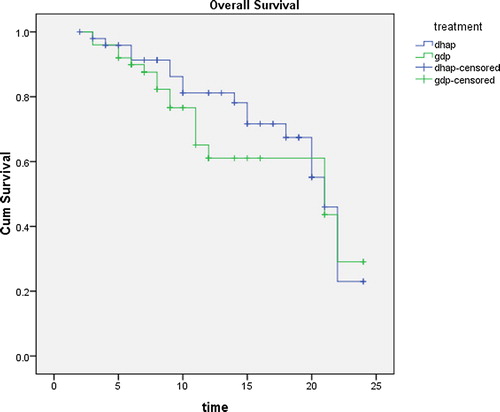
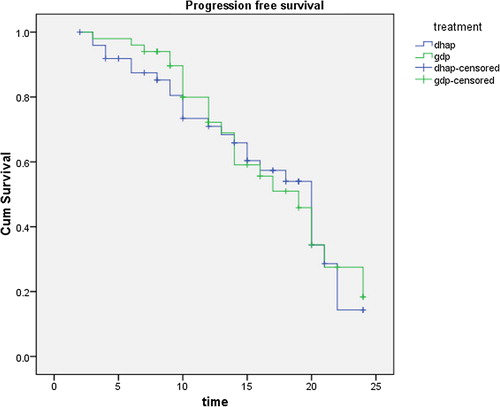
With a median follow up of patients of 14 months (2–24 months), there was no statistical significant difference between both groups regarding overall survival (OS) which was 30% and 25% in GDP and DHAP (p-value 0.4) . Also there was no statistical difference between both groups in progression free survival (PFS) which was 18% for GDP and 15% for DHAP (p value 0.8) ().
4 Discussion
Although salvage chemotherapy followed by ASCT is the standard of care in relapsed/refractory NHL,Citation7 in our country, due to limited resources, not all cases have that option. They only receive salvage chemotherapy.
So, in this study we tried to find a salvage regimen that has a low toxicity profile and better quality of life to be provided to this group of patients with poor prognosis.
DHAP regimen was used depending on the synergy demonstrated between cisplatin and cytarabine and it became widely used as a second line treatment in patients with relapsed or refractory lymphoma and was used as a standard treatment in the study done by Philip et al.Citation7
Gemcitabine is a pyrimidine antimetabolite which have structural similarities with cytarabine and is taken into cells more effectively. Gemcitabine containing regimens show activity and synergy and are used in relapsed refractory lymphoma.Citation8
This study included 100 NHL patients equally divided into 2 groups, one receiving GDP and the other receiving DHAP regimens. The primary endpoint in our study was the rate of response to treatment. Overall response was observed in 70% and 64% of patients receiving GDP and DHAP regimens with no statistical significant difference between both groups. These results correlated with study done by Crump M. et al.Citation9 who also found no statistical difference between patients receiving GDP and DHAP Their study included 619 patients with relapsed and refractory B &T cell- NHL but most patients in that study proceeded to ASCT after 2 cycles of salvage chemotherapy. They observed an ORR of 45% in GDP group and 44% in DHAP. Our results are slightly higher may be due to larger number of high risk patients in their study.
Another study done by Ismaeil et al.Citation10 found no significant difference between both regimens after 6 cycles of treatment. They included 62 patients with relapsed or refractory NHL and they observed an ORR of 67.6% in GDP and 65% in DHAP group. However, that study only included patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL).
Many other salvage chemotherapy regimens have been used in this group of patients with relapsed or refractory lymphoma and they resulted in good ORR as ICE and ESHAP regimens but they were associated a high toxicity profile with increased hospitalization rates and red cell and platelet transfusions.Citation3
Regarding toxicity profile, our study found no significant difference between both regimens in grade III–IV adverse events except in episodes of febrile neutropenia which occurred in 36% of patients in GDP group and 60% of patients in DHAP group (p-value 0.04). This correlated with results of Crump et al.Citation9 who found no significant difference between both groups regarding toxicity except for nausea and febrile neutropenia. However, Ismaiel et al.Citation10 found significant difference between both groups in febrile neutropenia, infection and stomatitis.
Hematologic toxicity remains a concern during treatment with second line chemotherapy regimens. In this study red cell transfusions were needed in 15% of patients receiving GDP and 25% receiving DHAP which is considered low when compared to other regimens as ICE (35%)Citation11 or mini BEAM (60%).Citation12 Fan et al observed the need of red cell transfusions in 9.2% of patients receiving GDP and platelet transfusions in 6.6% of patients.Citation13 In this study, platelet transfusions were needed in 12% and 15% in GDP and DHAP groups compared to 16.5% and 78% in ICECitation11 & mini BEAM.Citation12 Crump et al observed the need for platelet transfusions in 18% and 32% of patients receiving GDP and DHAP.Citation9
According to quality of life assessment, GDP was associated with improved quality of life than DHAP and the difference was highly significant.
Rituximab, a monoclonal antibody directed against CD20 exerts its antineoplastic effects through antibody dependant cellular cytotoxicity which results in decreased cellular proliferation. Adding Rituximab to different salvage chemotherapy regimens as DHAP or ICE has resulted in increased rates of complete remission and better progression free survival in patients who did not receive this drug in their primary regimen protocol. However, data regarding retreatment with rituximab are limited as a proportion of patients may develop resistance to the drug.Citation3
In conclusion, our results show that GDP can be used as a salvage treatment in patients with relapsed or refractory NHL with lower toxicity profile and better quality of life than DHAP but cannot be used as a standard of care without ASCT.
Limitations
The study has some limitations as small number of patients and unavailability of rituximab either in primary or salvage regimens.
Recommendations
Trials with larger number of patients and longer follow up are recommended.
Conflict of interest
No conflict of interest.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 25 July 2018
References
- N.H.WoodL.FellerE.J.RaubenheimerY.JadwatR.MeyerovJ.LemmerHuman immunodeficiency virus (HIV)-associated extranodal T cell non-Hodgkin lymphoma of the oral cavitySADJ: J S Afr Dental Assoc = tydskrif van die Suid-Afrikaanse Tandheelkundige Vereniging6332008158161
- Y.HouH.Q.WangY.BaRituximab, gemcitabine, cisplatin, and dexamethasone in patients with refractory or relapsed aggressive B-cell lymphomaMed Oncol294201224092416
- T.SeshadriJ.KuruvillaM.CrumpA.KeatingSalvage therapy for relapsed/refractory diffuse large B cell lymphomaBiol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant1432008259267
- B.D.ChesonS.J.HorningB.CoiffierReport of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working GroupJ Clin Oncol: Off J Am Soc Clin Oncol17419991244
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3. 0: development of a comprehensive grading system for the adverse effects of cancer treatment. In: Paper Presented At: Seminars in Radiation Oncology; 2003.
- C.S.BurckhardtK.L.AndersonThe Quality of Life Scale (QOLS): reliability, validity, and utilizationHealth Qual Life Outcomes1200360
- T.PhilipC.GuglielmiA.HagenbeekAutologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphomaN Engl J Med33323199515401545
- M.CrumpT.BaetzS.CoubanGemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG)Cancer1018200418351842
- M.CrumpJ.KuruvillaS.CoubanRandomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12J Clin Onco: Off J Am Soc Clin Oncol3231201434903496
- S.IsmaeilGemcitabine, dexamethasone, and cisplatin (GDP) versus dexamethasone, cytarabine, and cisplatin (DHAP) as salvage chemotherapy for patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL)Res Oncol.93–420139197
- C.H.MoskowitzJ.R.BertinoJ.R.GlassmanIfosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphomaJ Clin Oncol: Off J Am Soc Clin Oncol1712199937763785
- C.GirouardJ.DufresneK.ImrieSalvage chemotherapy with mini-BEAM for relapsed or refractory non-Hodgkin's lymphoma prior to autologous bone marrow transplantationAnn Oncol: Off J Eur Soc Med Oncol871997675680
- Y.FanZ.Y.HuangL.H.LuoH.F.YuEfficacy of GDP regimen (gemcitabine, dexamethasone, and cisplatin) on relapsed or refractory aggressive non-Hodgkin's Lymphoma: a report of 24 casesAi zheng = Aizheng = Chin J Cancer2711200812221225