?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: To compare various systems for assessing the risk of recurrent stones, based on the composition of urine.
Methods: The relative supersaturation (RSS) of urine, the Tiselius Indices, the Robertson Risk Factor Algorithms (RRFA) and the BONN-Risk Index were compared in terms of the numbers of variables required to be measured, the ease of use of the system and the value of the information obtained.
Results: The RSS methods require up to 14 analyses in every urine sample but measure the RSS of all the main constituents of kidney stones. The Tiselius Indices and the RRFA require only seven analyses. The Tiselius Indices yield information on the crystallisation potentials (CP) of calcium oxalate and calcium phosphate; the RRFA also provide information on the CP of uric acid. Both methods provide details on the particular urinary abnormalities that lead to the abnormal CP of that urine. The BONN-Risk Index requires two measurements in each urine sample but only provides information on the CP of calcium oxalate. Additional measurements in urine have to be made to identify the cause of any abnormality.
Conclusions: The methods that are based on measuring RSS are work-intensive and unsuitable for the routine screening of patients. The Tiselius Indices and the RRFA are equally good at predicting the risk of a patient forming further stones. The BONN-Risk Index provides no additional information about the causative factors for any abnormality detected.
Introduction
The incidence of upper urinary tract stone disease has increased steadily in most countries throughout the past 100 years and renal colic is now one of the commonest causes of emergency admission to hospital [Citation1]. The spontaneous passage of the stones or assisted passage through stenting or muscle relaxants (e.g. α-blockers [Citation2]), and various ‘minimally invasive’ procedures for stone removal or fragmentation, are the most frequently used means of alleviating the patient’s immediate problems. However, they do not cure the underlying abnormalities responsible for causing the stones to form and, consequently, patients often experience further episodes of stone formation if left without relevant prophylactic treatment. Classically, this involves metabolic screening followed by appropriate dietary and/or medical management to correct the abnormalities in urine composition for, ultimately, it is the composition of urine and the factors that control urine composition that determine whether or not a patient will form further stones.
There are several published methods for screening patients for their risk of forming stones. In the 1950s and 1960s, biochemical screening consisted of measuring plasma calcium and collecting a 24-h urine sample in which calcium and phosphate were generally the only constituents measured [Citation3]. A urine sample was often cultured to check for the presence of UTI, if this was suspected. Various simple quotients, such as the calcium/citrate and calcium/magnesium ratios, were devised which were claimed to discriminate between the urine of stone-formers and that of normal subjects, but none has stood the test of time. In 1969, a much more detailed method for estimating the chemical risk of forming crystals in urine was published that was based on calculating the level of supersaturation of the main stone-forming salts and acids in urine, using an iterative computer program called SUPERSAT [Citation4]. This was followed later by similar programs, e.g. EQUIL2 [Citation5], EQUIL93 [Citation6], SEQUIL [Citation7] and JESS [Citation8].
In the 1980s, Tiselius and Larsson [Citation9] devised a set of ‘Indices’ for assessing the biochemical risk of forming calcium-containing stones which greatly reduced the number of analytes required. For calcium oxalate (CaOx) the list of measurements was reduced to volume, calcium, oxalate, magnesium and citrate, and for calcium phosphate (CaP) the list consisted of volume, calcium, phosphate, citrate and pH [Citation10]. This reduced the total number of analytes required to measure the biochemical risk of forming the two Ca-containing salts in urine from 14 to seven.
During the same period, Robertson et al. [Citation11] published an alternative shortened method for calculating the biochemical risk of forming not only Ca-containing stones but also that of uric acid (UA). This also involved the measurement of only seven analytes in urine, in which phosphate in the Tiselius procedures was replaced by UA. These seven analytes were found to be the only urinary risk factors that were significantly different between a large group of idiopathic stone-formers and their age- and sex-matched controls. Using risk factor analysis and Bayes’ theorem, Robertson devised a set of five algorithms for calculating the biochemical risk of forming pure UA, mixed UA/CaOx, pure CaOx, mixed CaOx/CaP and pure CaP stones; this method was refined in 2003 [Citation12]. The technique was able to discriminate between untreated recurrent stone-formers and normal controls with ≈90% accuracy, and could predict the severity of the disease as defined by the number of stone episodes experienced by the patient per year if no prophylactic measures were prescribed to reduce stone risk. Comparison of the Tiselius Indices and the Robertson Risk Factor Analysis (RRFA) method showed a strong correlation between the systems in terms of their ability to predict the risk of forming Ca-containing stones (Robertson and Tiselius, unpublished results).
Within the last decade, Laube et al. [Citation13] reported the BONN-Risk Index for assessing the risk of urine samples forming CaOx crystals. This required only two measurements in urine, i.e. the concentration of ionised calcium (Ca2+) and the amount of oxalate that must be titrated into the urine to cause it to form crystals of CaOx.
Methods
The various methods for assessing the risk of forming crystals of the various stone-forming salts and acids in urine were compared, taking into account: (i) the number of measurements required; (ii) the information generated by the procedure; (iii) the advantages; and (iv) disadvantages of each system.
Results
shows a comparison of the various methods for assessing the risk of forming crystals in urine and this, in turn, is presumed to reflect the risk of the patient forming further stones. The results show the following:
Table 1 Comparison of the various methods for assessing the risk of crystalluria and recurrent stone formation.
The methods for calculating the relative supersaturation (RSS) of urine require the measurement of up to 14 analytes in every urine sample, and although they provide new information about the risk of crystal formation of all the main stone constituents in urine, none is suitable for routine use in a Stone Clinic as they are all expensive and time-consuming to run. Moreover, they do not take into account the effects of the various inhibitors and promoters that are known to influence the rate of crystallisation and agglomeration of the resulting crystals.
The Tiselius Indices and the RRFA both require seven analytes in every urine sample. The Tiselius Indices provide information on the overall crystallisation potential (CP) of urine with respect to CaOx and CaP, taking into account the risk of new crystal formation and the growth of existing crystals. The RRFA provide information on the overall CP of urine with respect to CaOx, CaP and UA (and various mixtures of these), taking into account the risk of new crystal formation and the growth of existing crystals. Neither procedure provides any information on the CP of urine with respect to magnesium ammonium phosphate (MAP) or cystine.
The BONN-Risk Index requires two measurements in every urine sample but only provides information on the crystallisation potential of CaOx in urine that has been previously filtered to take out any pre-existing crystals. The value of this procedure in assessing the CP of the original urine is therefore debatable, as the urine composition might have changed as a result of the removal of any CaOx crystals. Moreover, the procedure provides no information on the CP of urine with respect to any of the other stone-forming salts and acids.
Discussion
Although the above procedures can be useful to varying extents in defining patient’s risk of forming further stones, it is usually necessary to carry out further tests to identify the cause(s) of the urinary abnormalities uncovered during the above procedures performed on the 24-h urine samples. It is usually best to carry out such investigations at a time when the patients are eating and drinking ‘normally’ in their free, home environment. The tests should not be conducted if the patient has haematuria or immediately before or just after stone removal. Once the stone removal procedure has been carried out, it is advisable to wait for at least 2 months before carrying out the screening tests, as during that period the patients often consume a diet that is different from that before their stone episode, and the risk of stone recurrence might be ‘artificially’ or temporarily low, i.e. the so-called ‘Stone Clinic effect’ described by the Mayo Clinic group [Citation14]. If the patients do not form another stone within 3 months of the current episode, they frequently return to their former ‘bad’ dietary and lifestyle habits, and the risk of stones again increases [Citation15]. The biochemical screening should never be carried out when the patient is in hospital, as the hospital diet is likely to be very different from that consumed by the patient on his/her freely chosen home diet, and this will confuse the picture.
As urolithiasis is a multifactorial problem there is no simple approach to the biochemical screening of patients. Not only is there a wide range of urinary biochemical factors to be considered, there are also other determinants that might lead to stones as a secondary problem, such as certain metabolic disorders, a family history of stones and/or of other medical disorders, and a range of epidemiological, demographic, genetic, nutritional and lifestyle factors.
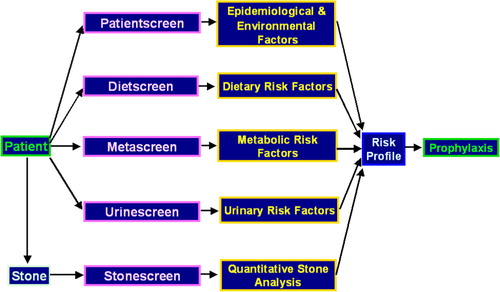
In the London Centre for Kidney Stone Research a comprehensive screening procedure has been developed over the past 13 years [Citation16] for investigating patients with stones. This takes into account the recommendations of several working parties on the management of patients with stones [Citation17–Citation19]. The screening system consists of the following tests, shown diagrammatically in .
Patient history screen
A complete demographic, lifestyle and medical history of the patient is recorded including:
| • | Date of birth, gender, weight, height and body mass index. | ||||
| • | Blood pressure. | ||||
| • | Ethnic origins including details of any cultural or religious habits or practices. | ||||
| • | A detailed stone episode history, including the age at onset of stones. | ||||
| • | A medical history not only of stones but also of other relevant disorders that might lead to stones as a secondary problem. | ||||
| • | A history of surgical procedures that might lead to stones as a secondary problem. | ||||
| • | A lifestyle questionnaire including details of occupation, working or living in a hot environment, night-time sweating, strenuous exercise that leads to sweating, air travel (which is also a dehydrating situation) and recent vacations in a tropical setting. | ||||
| • | A history of UTIs. | ||||
| • | Details of any anatomical abnormalities in the urinary tract. | ||||
| • | A history of past and current medications. | ||||
| • | A family history of stones. | ||||
| • | A family history of other medical problems that might influence the risk of stone formation, such as hypertension, type 2 diabetes, bowel disease and any inborn errors of metabolism that are associated with stone formation. | ||||
Table 2 The metabolic factors involved in secondary UA and calcium stone formation and their effects on urinary risk factors and stone type.
The detailed patient history also allows the identification of lifestyle factors that might increase the risk of forming stones from the regular passage of concentrated urine. Factors ascertained include whether the patient perspires a lot normally (particularly at night) or exercises strenuously and fails to sufficiently replenish water losses, or whether the patient lives in a hot environment or travels to the Tropics on holiday or business. The occupation of the patient is also important: working in a hot or other dehydrating environment, e.g. chefs or kitchen-workers in restaurants, or airline pilots and air-crew; an intensive job where it is difficult to go to the toilet (and so they do not drink enough) such as taxi-drivers or surgeons; working as an over-pressurised business executive who professes, ‘to be too busy to stop and have a drink or go to the toilet’; working with metals or other chemicals that can damage the kidneys, e.g. cadmium, beryllium and toluene, and so lead to a secondary metabolic acidosis. Also identified are ethnic and other habits that can lead to an increase in stone formation, e.g. as betel-chewing; or regularly consuming antacids that contain high amounts of calcium and alkali; laxative abuse; or likely to form iatrogenic stones consisting of silica, from the regular ingestion of magnesium trisilicate antacids; or stones consisting of other drugs or their metabolites such as sulphonamide, triamterene, sulphadiazine or indinavir. A detailed list of the lifestyle factors that can lead to stone formation is given in .
Stone-analysis screen
All patients and the members of the Lithotripsy Units, Endourological Departments and urologists involved with the patients concerned should be strongly encouraged to retain the stones or stone fragments for analysis after passage or removal. The stones should then be analysed quantitatively by Fourier transform infra-red spectroscopy [Citation20], an important tool as it provides an additional clue as to the cause of the stone(s) in the patient concerned. Unfortunately, the collection of stones is not well organised in many hospitals and, in any case, most do not have the facilities for the quantitative analysis of the stones.
Metabolic screen
This provides a metabolic assessment of the patient in terms of identifying disorders that are likely to lead to the formation of secondary stones in the urinary tract [Citation16]. This requires a blood and urine sample to be taken from the patient at their first appointment at the Stone Clinic, preferably after an overnight fast, and analysed for the following:
Blood: Urea, creatinine, sodium, potassium, bicarbonate, chloride, albumin, calcium, corrected calcium, magnesium, phosphate, alkaline phosphatase, UA, glucose, parathyroid hormone and 25-hydroxyvitamin D3. Plasma oxalate might also be required in patients with suspected hereditary hyperoxaluria, but special precautions have to be taken in the sample collection and handling for this analysis.
Urine: Osmolality, urea, creatinine, sodium, potassium, calcium, phosphate, magnesium, UA, oxalate and pH (measured by pH meter, not by dipstick). A sterile sample of urine should be sent for microbiological testing in cases of suspected UTI. In the light of current interest in the role of ‘metabolic syndrome’ and UA stone formation, it can also be useful to measure titratable acidity, ammonium ion concentration () and bicarbonate, to assess the net acid excretion (NAE) of the patient.
From these analyses, it is possible to determine whether or not the patient has any underlying metabolic disorder that might lead to stones, such as primary hyperparathyroidism, distal renal tubular acidosis, Dent’s disease, hereditary or enteric hyperoxaluria, hyperuricaemia, hypomagnesaemia, metabolic syndrome, hypervitaminosis D or UTI. A full list of all the main metabolic disorders or conditions that might lead to secondary stone formation and the risk factors that lead to stones is given in .
It is also possible to determine the renal handling of ions that are particularly involved in the formation of stones, e.g. calcium, phosphate, UA, magnesium, citrate and oxalate. Patients with renal leaks of calcium and/or phosphate can be identified and the cause of the renal leak related to their parathyroid hormone and 25-hydroxyvitamin D3 status, and to their renal throughputs of Na+ and H+ ions, high levels of which are known to cause calcium to leak out through the kidneys. Renal leaks of other stone-forming constituents of urine, e.g. UA, can also be identified.
Urine screen
This consists of two 24-h urine samples collected on consecutive days, the first into a 5-L plastic bottle containing 50 mL of 2.2 M hydrochloric acid as a preservative. (Note: Suitable warnings have to be given to the patient regarding the safe management of their acid-containing bottles.) This acidified urine collection is analysed for volume, creatinine, calcium, magnesium, oxalate, citrate and urea. On the following day, a second 24-h collection is made into a plain 5-L container and analysed for volume, creatinine, pH (measured accurately by a pH meter, not by dipstick), sodium, potassium, urate, protein, and a qualitative test for cystine (then quantitative for cystine, lysine, ornithine and arginine if the qualitative test for cystine is positive).
From the combined analyses of these two urine samples, several algorithms can be used to assess the overall biochemical risk (PSF) of forming stones containing UA, CaOx or CaP, or various mixtures of these constituents [Citation12]. This requires the use of seven of the above measurements in the 24-h urine samples (volume and pH, and the urinary excretions of calcium, magnesium, oxalate, citrate and UA, as mentioned earlier). The PSF values are calculated on a probability scale from 0 to 1. Values of >0.5 are indicative of a significant risk of forming stones; values of >0.9 are often found in urine from actively recurrent stone-formers. In most patients the high risk is rarely due to a single abnormal urinary constituent (except in the case of primary hyperoxaluria) but is more commonly due to a combination of between two and seven lesser ‘abnormalities’, depending on the stone type. Indeed, it is possible to have a high PSF value with every urinary risk factor within its ‘normal range’ but with several of the risk factors lying towards the upper or lower limits of these ranges. This is an important feature of the model, as it allows a risk assessment to be made of the patient who would have been previously described as ‘having no obvious abnormalities in his/her urine’ yet has an abnormal combination of the variables that lead to crystalluria and stones . shows that the Tiselius Index for CaOx also identifies the urine of the stone former to be abnormal. Both models of stone formation can be used not only to assess patient’s probability of forming stones before treatment, but also to follow his/her progress during the follow-up programme of stone prophylaxis.
Table 3 An example of risk accumulation in an otherwise ‘normal-looking’ urine sample from a CaOx/CaP stone former vs. that in a normal subject with a similar, but a lower-risk, urinary composition.
Nutritional screen
During the week preceding the collection of the 24-h urine samples the patient is requested to complete a diet diary of everything that is consumed each day on a freely chosen home diet. The patient should be asked not to change their diet as this will only serve to confuse the screening procedure. The last 2 days of the diet diary should correspond to the 2 days of the 24-h urine collections. The diary is analysed for total fluid intake (including what is contained in the various foodstuffs), calories, total calcium and calcium derived from dairy products, magnesium, sodium, potassium, phosphate, oxalate, purine, total protein (and its various fractions, including animal protein, meat + fish + poultry protein, dairy protein, and fruit + vegetable + cereal protein), fibre, fat, refined sugars and potential renal acid load (PRAL). The diet screen allows an assessment of the role of diet in patient’s risk of stones, and can usually be correlated with the composition of the 24-h urine samples and the metabolic and clinical status of the patient as determined above.
The PRAL can be used to estimate the NAE of the patient. From this, an estimated 24-h urinary pH can be determined from previously published relationships between urinary pH and NAE [Citation21]. The expected urinary pH estimated in this way can then be compared with the actual pH measured in the plain 24-h urine sample described above. This can help in the diagnosis of acid–base disorders, such as distal renal tubular acidosis, and renal buffering disorders, such as metabolic syndrome [Citation22]. It can also identify those patients who had a UTI at the time of the investigations.
Epidemiological factors in the formation of urinary stones
There are three groups of epidemiological factors that have been found to be important in the formation of urinary stones, i.e. demographic, environmental and pathophysiological. Each of these groups contains several categories, summarised in and . Each has been shown to increase the risk of stone formation through its effect on the balance between supersaturation, inhibitors and promoters of crystallisation in urine. For calcium stone formation, the most common form of the disorder, the main epidemiological factors are age, gender, season, climate, stress, occupation, affluence, diet (including fluid intake) and various genetic/metabolic factors. The role of diet in particular has been studied in detail, and this appears to explain much of the changing pattern of stone incidence over the past 100 years. As the composition of the diet becomes ‘richer’ in a given population (through an increased consumption of protein, particularly animal protein, refined sugars and salt), the incidence of upper urinary tract stones is found to increase. This often coincides with periods of economic expansion. However, during periods of economic recession the incidence of stones has been noted to decrease in parallel with a return to a more healthy and usually more vegetarian form of diet containing more fibre, more magnesium and potassium, and fewer energy-rich foods.
Table 4 The main demographic and lifestyle factors involved in UA and calcium stone formation, and their effects on urinary risk factors.
Prevention of stone recurrence
The main aim in preventing stone recurrence is to decrease the likelihood of crystals forming in the urinary tract by reducing the supersaturation of urine with respect to the particular constituent(s) that occur in patients’ stones. Alternatively, it is sometimes possible in patients who initially have low protection against forming Ca-containing stones to increase their urinary excretions of citrate and magnesium, which will reduce the rate of crystallisation and degree of agglomeration of any Ca-containing crystals formed in patients’ urine. The net effect of changes in urinary composition produced by the treatment regimen can be monitored using the urine-screen test and the determination of the biochemical risk of stone formation using either the Tiselius Indices or the RRFA.
Conflict of interest
No conflict of interest to declare.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- W.G.RobertsonThe medical management of urinary stone diseaseEur Urol Update Ser71998139144
- J.M.HollingsworthM.A.RogersS.R.KaufmanT.J.BradfordS.SaintJ.T.WeietalMedical therapy to facilitate urinary stone passage: a meta-analysisLancet368200611711179
- A.HodgkinsonL.N.PyrahThe urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal originBr J Surg4619581018
- W.G.RobertsonMeasurement of ionized calcium in biological fluidsClin Chim Acta241969149157
- P.G.WernessC.M.BrownL.H.SmithB.FinlaysonEQUIL2. A BASIC computer program for the calculation of urinary saturationJ Urol134198512421244
- C.M.BrownD.K.AckermannD.L.PurichEQUIL93: a tool for experimental and clinical urolithiasisUrol Res221994119126
- R.A.AshbyJ.P.ByrneA.Z.GyöryUrine is a saturated equilibrium and not a metastable supersaturated solution: evidence from crystalluria and the general composition of calcium salt and uric acid calculiUrol Res271999297305
- P.MayK.MurrayJESS, a joint expert speciation system 1Talanta38199114091417
- H.G.TiseliusL.LarssonBiochemical evaluation of patients with urolithiasisEur Urol719813134
- H.G.TiseliusA simplified estimate of the ion-activity product of calcium phosphate in urineEur Urol101984191195
- W.G.RobertsonM.PeacockP.J.HeyburnD.H.MarshallP.B.ClarkRisk factors in calcium stone disease of the urinary tractBr J Urol501978449454
- W.G.RobertsonA risk factor model of stone-formationFront Biosci8200313301338
- N.LaubeA.SchneiderA.HesseA new approach to calculate the risk of calcium oxalate crystallization from unprepared native urineUrol Res282000274280
- D.H.HoskingS.B.EricksonC.J.Van den BergD.M.WilsonL.H.SmithThe stone clinic effect in patients with idiopathic calcium urolithiasisJ Urol130198311151118
- R.W.NormanS.S.BathW.G.RobertsonM.PeacockWhen should patients with symptomatic urinary stone disease be evaluated metabolically?J Urol132198411371139
- W.G.RobertsonA comprehensive screening procedure for the assessment of patients with recurrent stonesL.BorghiT.MeschiA.BrigantietalKidney stones1999Editoriale BiosCosenza407410
- H.G.TiseliusD.AckermannP.AlkenC.BuckP.ConortM.GallucciWorking party on lithiasis, European Association of Urology. Guidelines on urolithiasisEur Urol402001362371
- M.StraubW.L.StrohmaierW.BergB.BeckB.HoppeN.LaubeetalDiagnosis and metaphylaxis of stone disease. Consensus concept of the National Working Committee on Stone Disease for the upcoming German Urolithiasis GuidelineWorld J Urol232005309323
- G.M.PremingerH.G.TiseliusD.G.AssimosP.AlkenC.BuckM.GalluccietalEAU/AUA Nephrolithiasis Guideline PanelJ Urol178200724182434
- G.P.KasidasC.T.SamuellT.B.WeirRenal stone analysis. Why and how?Ann Clin Biochem4120049197
- T.RemerF.ManzPotential renal acid load of foods and its influence on urine pHJ Am Diet Assoc951995791797
- W.G.RobertsonD.NairC.LaingS.ChoongP.JaegerR.J.UnwinThe role of ‘Metabolic Syndrome’ in the formation of uric acid-containing stonesUrol Res362008177