?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: To compare the effect of ischaemic preconditioning (Ipre) vs. ischaemic postconditioning (Ipost) on renal ischaemia/reperfusion (I/R) injury in rats.
Materials and methods: In all, 120 male Sprague–Dawley rats were classified into four groups of 30 rats each, designated sham, control, Ipre and Ipost. Renal function, including serum creatinine, blood urea nitrogen (BUN), creatinine clearance (CrCl), fractional Na excretion (FENa) and renal histopathology were measured at 2, 24 and 48 h after ischaemia. Markers of lipid peroxidation (malondialdehyde, MDA), superoxide dismutase (SOD) and reduced glutathione (GSH) were measured in kidney tissues during the same intervals.
Results: Ipre caused a significant improvement in renal function, as indicated by a significant decrease in serum creatinine, BUN and FENa, with a significant increase in CrCl. However, Ipost caused no significant improvement in renal function. Morphologically Ipre caused a marked significant improvement in the renal tubular damage score compared to Ipost. Also, Ipre caused a significant decrease in MDA, and significant increase in GSH and SOD when compared to Ipost.
Conclusion: Ipre is more potent than Ipost for improving the renal injury induced by I/R. Ipre caused a marked improvement in renal function and morphology, while Ipost caused a minimal improvement in morphology only. Moreover, Ipre caused a marked and significant reduction in oxidative stress in kidney tissues, while Ipost caused a minimal reduction.
Abbreviations:
- Ipre
- ischaemic preconditioning
- Ipost
- ischaemic postconditioning
- I/R
- ischaemia/reperfusion
- BUN
- blood urea nitrogen
- CrCl
- creatinine clearance
- FENa
- fractional Na excretion
- MDA
- malondialdehyde
- SOD
- superoxide dismutase
- GSH
- reduced glutathione
- ROS
- reactive oxygen species
- H&E
- haematoxylin and eosin
- OSOM
- outer stripe of the outer medulla
Introduction
Renal ischaemia/reperfusion (I/R) injury is a common cause of acute renal failure and contributes to considerable morbidity associated with surgery and anaesthesia [Citation1, Citation2] . Although several decades of research have greatly improved the understanding of the mechanisms underlying renal I/R injury, effective drugs for treating it are still unavailable. Therefore, it is necessary to actively explore other approaches for this problem. Ischaemic preconditioning (Ipre), a well-established phenomenon that describes tissue adaptation to stress by exploiting intrinsic defence mechanisms, was initially described in the heart by Murry et al. [Citation3] and Ambros et al. [Citation4]. Ipre consists of transient periods of non-lethal ischaemia before a subsequent lethal episode of ischaemia. In 2003, Zhao et al. [Citation5], introduced the concept of ischaemic postconditioning (Ipost), which consists of one or more short cycles of reperfusion followed by one or more short cycles of ischaemia, immediately after an ischaemic phase and before the permanent reperfusion.
The efficacy of the protective effect of Ipre and Ipost is variable. In the heart, some studies showed no statistically significant difference in the reduction of infarct size for Ipre and Ipost [Citation5], while other studies showed Ipre to be more effective than Ipost [Citation6]. The differences between the efficacy of these cardioprotective methods might suggest differing underlying protective mechanisms. In the small intestine, dos Santos et al. [Citation7] recently concluded that Ipre and Ipost were equally able to minimise the tissue injury in the intestines of rats subjected to mesenteric ischaemia and reperfusion. In the kidney, several studies reported the beneficial effect of Ipre [Citation8–Citation11] and Ipost [Citation12, Citation13] on renal I/R injury in different species of animal. Nevertheless, to the best of our knowledge, no study has been designed to compare the magnitude of the renoprotective effects of Ipre and Ipost against renal I/R injury. Thus the aim of the present study was to compare the efficacy of Ipre and Ipost in protecting against renal I/R injury in a rat model, for both renal function and renal morphology, and to compare their effects on the redox state in kidney tissue.
Materials and methods
The study included 120 male Sprague–Dawley rats (body weight 200–250 g, 4–6 months old) that were bred in the animal research facility in the Urology & Nephrology Centre at Mansoura, Egypt. Experiments were performed according to the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, National Research Council, Washington, DC: National Academy Press, No. 85–23, revised 1996). All protocols were approved by our ethical committee of Mansoura, Faculty of Medicine.
Study design
The rats were randomly divided into four equal groups: (1) sham, where rats were subjected to right nephrectomy and exposure of the left renal pedicle with no ischaemia; (2) controls, subjected to right nephrectomy and left renal ischaemia for 45 min (definitive ischaemia); (3) Ipre, treated as the control group, but preconditioning ischaemia was induced before the definitive ischaemia; (4) Ipost, treated as the control group, but Ipost was induced after the definitive ischaemia. Each group was subdivided into three subgroups each contains 10 rats, that were killed humanely at 2, 24 and 48 h, respectively, according to the designated subgroup, and the kidneys harvested. Blood and urine samples were collected just before the death. Urine could not be collected at the 2-h sample time.
Experimental model
For the sham operation the rats were anaesthetised with a mixture of ketamine 75 mg/kg and diazepam 5 mg/kg intraperitoneally. After inducing anaesthesia a midline laparotomy was made, then a right nephrectomy was done and the left kidney and its pedicle were dissected off the surrounding perirenal fat along the renal surface. The left kidney was exposed for 45 min with no vascular clamping. The abdomen was then irrigated with isotonic saline and the abdominal incision closed by continuous suture using polyglactin 2/0.
The control group was treated as the sham group but the left renal artery was clamped using a vascular clip for 45 min and the right nephrectomy was done 5 min before removing the vascular clamp.
The Ipre group was also treated like the control group, but three cycles of 2 min of ischaemia, followed by a 5-min reperfusion period for I/R, were applied before the 45 min of ischaemia [Citation4].
The Ipost group was also treated like the control group, but 3, 6 and 12 min of reperfusion were applied consecutively, separated by 5 min of re-occlusion, after 45 min of ischaemia [Citation14].
Assessment of renal function
Serum creatinine and blood urea nitrogen levels (BUN) were estimated from blood samples. Endogenous creatinine clearance (CrCl), and fractional Na excretion (FENa+) were calculated as, respectively;and
Renal oxidative stress after IR injury was determined by measuring renal cortical malondialdehyde (MDA, an index for lipid peroxidation) and superoxide dismutase (SOD, an endogenous antioxidant enzyme).
Histopathological examination
Explanted kidneys were bisected along the long axis and were fixed in 10% formalin solution for 24 h. After automated dehydration through a graded-alcohol series, transverse kidney slices were embedded in paraffin, sectioned at 4 μm, and stained with haematoxylin and eosin (H&E). For the histopathological assessment of ischaemic tubular injury we used a modified form of well-established grading scales (scores of 0–4) [Citation15]. The numerical score used was as follows: 0, no damage; (1) unicellular patchy isolated necrosis; (2) tubular necrosis <25%; (3) tubular necrosis 25–50%; and (4) >50% tubular necrosis.
One-way anova was used to assess the significance within treated groups, within Scheffe’s posthoc test, and P < 0.05 was taken to indicate statistical significance.
Results
In basal conditions all the groups had comparable values for all the variables of renal function. CrCl and FENa were not calculated at 2 h after ischaemia because urine could not be collected at that time. Compared to the sham group, the control and study groups had a significant increase in serum creatinine, BUN and FENa, and a significant decrease in CrCl at all sample times (P < 0.05). Nevertheless, the percentage change in the Ipre group was significantly less than in the Ipost group. Compared to the control group, the Ipost group had no significant change at all sample times, but the Ipre group had significant decreases in serum creatinine, BUN and FENa, and a significant increase in CrCl at 24 and 48 h after ischaemia (P < 0.05; ).
Table 1 The effect of Ipre and Ipost on the variables of renal function, from 10 rats at each sample time.
Oxidative stress
Compared to the sham group the control group had a significant increase in MDA and a significant decrease in GSH and SOD. Compared to control group the study groups had a significant decrease in lipid peroxide (MDA) concentration (P < 0.05). Nevertheless, these changes were more significant in the Ipre than the Ipost group. Also, compared to control group, the study groups had significant increases in the concentration of SOD and GSH (P < 0.05). These changes in concentrations were more significant in the Ipre than the Ipost group ().
Table 2 The effect of Ipre and Ipost on lipid peroxidation products (MDA), and the antioxidants SOD and GSH.
Renal morphology
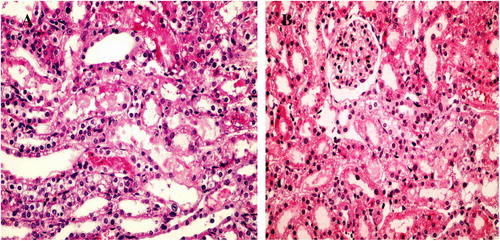
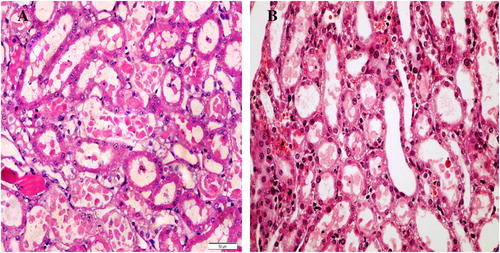
Kidneys from the control rats showed a significant increase in tubular injury score (scores 3, 4) when compared with that of sham rats (P < 0.05; ). The kidney sections from the sham group showed the normal preserved kidney structure (A). For the control group, the most severe and pronounced injury was in the cortex and the outer stripe of the outer medulla (OSOM), with a pattern of acute tubular necrosis, which included widespread degeneration of the tubular architecture, detachment of epithelial cells from the basement membrane, tubular cell necrosis, intratubular cast formation and luminal congestion with extensive loss of the brush border (B). Renal sections from the Ipre group showed a marked reduction in the histological features of renal injury (A and B), consisting of mild individual tubular necrosis and minimal tubular dilatation (scores 1, 2, of which 17 rats had score 1 and 13 had score 2) when compared with the control group in the same period (P < 0.05). However, in the Ipost group there were no significant changes (A and B) compared with the control group.
Figure 1 The effects of Ipre and Ipost on the tubulo-interstitial damage score at 2, 24 and 48 h after ischaemia in the different groups. Significant difference from the ∗sham group, #control group, and $significant from the Ipre group. One-way anova with Scheffe’s posthoc test (P < 0.05).
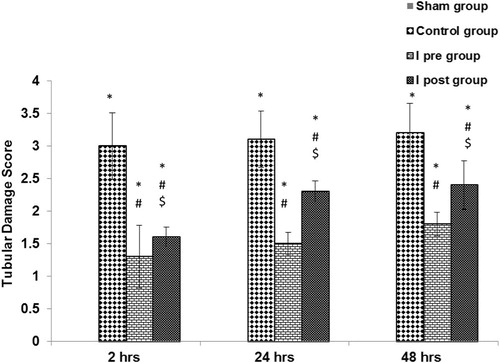
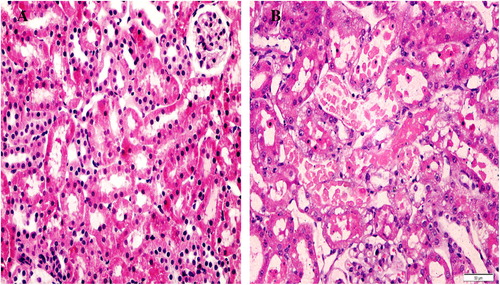
Figure 2 Sections of the kidney from the sham group (A), showing a normal appearance of glomeruli and tubules (score 0), and the control group (B), showing tubular necrosis at OSOM in >50% of tubules, score 4. H&E ×400).
Discussion
Ipre and Ipost are simple methods to render an organ more tolerant to ischaemia [Citation4, Citation16] . Also, the critical role of reactive oxygen species (ROS) in I/R-mediated renal injury has been shown by many studies [Citation17–Citation19] and a reduction of oxidative stress might be one of the potential mechanisms of the renoprotective effect of Ipre and Ipost on renal I/R injury. Thus, in the present study, we compared the effect of Ipre and Ipost on renal function and morphology, and on oxidative stress in renal I/R injury. Ipre significantly improved renal function at the tubular and glomerular levels, as indicated by a significant increase in CrCl, and significant decreases in serum BUN, creatinine and FENa in the Ipre compared with the control group at 2, 24 and 48 h after renal ischaemia. These findings are in agreement with those from previous studies Citation21] . Unfortunately, Ipost caused no significant improvement in renal function at 2, 24 and 48 h after renal ischaemia. These findings are consistent with those reported by Zhuang et al. [Citation22] and not in line with those of Szwarc et al. [Citation12], who reported that Ipost caused a significant improvement in renal function after renal I/R injury. Moreover, in a recent study, Weng et al. [Citation23] reported that Ipost inhibited tubulointerstitial fibrosis after renal ischaemia. They used a rat model of 45-min of ischaemia and reperfusion for 12 weeks. The present findings suggest that Ipre is more effective than Ipost in renal protection from I/R injury.
It was reported that not all combinations and durations of ischaemia and reperfusion trigger the preconditioning phenomenon to protect the kidney from I/R injury [Citation10]. Previous studies indicated that left renal artery occlusion for four cycles of ischaemia for 4 min with a 12-min reperfusion, or Ipre with four cycles of renal ischaemia for 8 min with a 5-min reperfusion, or bilateral renal pedicle occlusion for three cycles of ischaemia for 5 min followed by 5 min of re-flow, protected renal function and improved renal morphology after subsequent severe I/R injury [Citation7–Citation9] . In the present study the findings were similar, although we used three cycles of 2 min of ischaemia and 5 min of reperfusion. Also, Torras et al. [Citation10] and Jia et al. [Citation11] reported that one cycle of 15 min of ischaemia and 10 min of reperfusion was the optimum Ipre schedule for a renal isograft.
Renal I/R injury is a complex inflammatory process characterised by tubular epithelial cell necrosis and apoptosis, marked monocyte infiltration, and increased production ROS. The latter act as a key mediator in the renal injury induced by I/R, and cellular defence mechanisms against oxidative stress are necessary to maintain normal cellular function. In the present study there was a lower concentration of GSH and SOD in the control group that was enhanced by Ipre. Also, the level of MDA (a marker of lipid peroxidation) increased significantly in kidney tissues after renal I/R, and Ipre reversed the increase in lipid hydroperoxide levels to a considerable extent. These findings suggest that an attenuation of oxidative stress in the kidney might be one of the potential protective mechanisms of Ipre.
Although previous studies reported that Ipost provides a renoprotective effect on I/R-induced renal injury in rat and mice [Citation13], we failed to detect any protective effect of Ipost on I/R injury in this rat model, at the least at the level of renal function, which showed no significant improvement in the Ipost group when compared to the controls. Nevertheless, the Ipost group showed a significant improvement in renal tubular damage score. Consistent with the present study, the ineffectiveness of Ipost was also reported by Zhuang et al. [Citation22], but they did not report improvement at the level of renal function and morphology, and the present study failed to detect any improvement in renal function only. In cardiac muscle, Schwartz et al. [Citation24] showed that 30-s cycles of repetitive ischaemia during reperfusion has no protective effect on pig hearts subjected to lethal ischaemia. Also, Dow et al. [Citation25] showed that Ipost does not reduce myocardial infarct size in an in vivo regional-ischaemia rodent model.
The discrepancy in the effectiveness of Ipost on renal I/R injury between the present and previous studies remains unknown, but it might be due to differences in the duration of ischaemia and of Ipost in these studies. In the present model the ischaemia time was 45 min and cycles of Ipost were three of 5 min of ischaemia separated by 3, 6 and 12 min of reperfusion, so the total ischaemia time was 60 min. However, in the study of Szwarc et al. [Citation13] it was 30 min followed by three cycles of 30 s of ischaemia separated by 30 s of reperfusion. Also, Zhuang et al. [Citation22] showed in mice that left renal ischaemia for 26 min followed by 30 s of ischaemia separated by 30 s of reperfusion did not improve the renal injury induced by I/R. Also, in the present study, the Ipost showed a significant decrease in oxidative stress, as indicated by a significant decrease in MDA, and significant increases in SOD and GSH in the Ipost group when compared to controls. However, the effect of Ipost was less significant than for Ipre. Hence the protective effect of Ipost might be due to a reduction in oxidative stress.
The present study, to the best of our knowledge, is the first to compare the effect of Ipre and Ipost on renal I/R injury. However, the study has some limitations, e.g., the mechanisms to explain the renoprotective effects of Ipost and Ipre are insufficient. Further studies are needed to resolve the controversy in Ipost results in the present and previous studies. We recommend studying the effect of Ipre and Ipost on the activation and expression of antioxidant genes, such as Nrf2, and on inflammatory cytokines and the apoptotic process.
In conclusion, Ipre ameliorated the renal injury induced by renal I/R, while Ipost was associated with no significant improvements in renal function. However, both treatments attenuated the tubular damage score and oxidative stress in kidney tissues, but the effect of Ipre was more potent than Ipost. Further studies are needed to understand the underlying mechanisms of these effects.
Conflict of interest
None.
Source of Funding
This work was funded through the Science and Technology Development Fund (STDF), Egypt-grant no. 1146.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- V.ChanderK.ChopraProtective effect of nitric oxide pathway in resveratrol renal ischemia-reperfusion injury in ratsArch Med Res3720061926
- S.AronsonR.BlumenthalPreoperative renal dysfunction and cardiovascular anaesthesia: concerns and controversiesJ Cardiothorac Vasc Anesth171998117130
- C.E.MurryR.B.JenningsK.A.ReimerPreconditioning with ischemia. A delay of lethal cell injury of lethal cell injury in ischemic myocardiumCirculation74198611241136
- J.T.AmbrosI.Herrero-FresnedaO.G.BorauJ.M.BoiraIschemic preconditioning in solid organ transplantation: from experimental to clinicsTransplant Int202007219229
- Z.Q.ZhaoJ.S.CorveraM.E.HalkosF.KerendiN.P.WangR.A.GuytonetalInhibition of myocardial injury by ischemic postconditioning during reperfusion comparison with ischemic preconditioningAm J Physiol Heart Circ Physiol2852003H579H588
- L.ArgaudO.Gateau-RoeschO.RaiskyJ.LoufouatD.RobertM.OvizePostconditioning inhibits mitochondrial permeability transitionCirculation1112005194197
- C.H.dos SantosO.M.GomesJ.C.PontesL.N.MiijiM.A.BispoThe ischemic preconditioning and postconditioning effect on the intestinal mucosa of rats undergoing mesenteric ischemia/reperfusion procedureActa Cir Bras2320082228
- N.ToosyE.L.McMorrisP.A.GraceR.T.MathieIschaemic preconditioning protects the rat kidney from reperfusion injuryBJU Int841999489
- H.T.LeeC.W.EmalaProtective effects of renal ischemic preconditioning and adenosine pretreatment: role of A (1) and A (3) receptorsAm J Physiol Renal Physiol2782000F380
- J.TorrasI.Herrero-FresnedaN.LloberasPromising effects of ischemic preconditioning in renal transplantationKidney Int61200222182227
- R.P.JiaJ.J.XieF.Y.LuoJ.G.ZhuIschemic preconditioning improves rat kidney allograft function after ischemia/reperfusion injury role tumor necrosis factor-alphaTransplant Proc40200833163320
- S.SzwarcN.SoullierN.GayrardC.MejeanG.MouradA.ArgilesIschemic postconditioning prevents ischemic acute renal failureTransplant Proc3920072554
- X.LiuH.ChenB.ZhanB.XingJ.ZhouH.ZhuetalAttenuation of reperfusion injury by renal ischemic postconditioning: the role of NOBiochem Biophys Res Commun3592007628634
- G.ServiddioA.D.RomanoL.GesualdoR.TamborraA.M.Di PalmaT.RolloetalPostconditioning is an effective strategy to reduce renal ischaemia/reperfusion injuryNephrol Dial Transplant23200815041512
- K.SolezE.C.KramerJ.A.FoxR.H.HeptinstallMedullary plasma flow and intravascular leukocyte accumulation in acute renal failureKidney Int619742437
- J.Vinten-JohansenZ.Q.ZhaoA.J.ZattaH.KinM.E.HalkosF.KerendiPostconditioning: new link in natures armor against myocardial ischemia-reperfusion injuryBasic Res Cardiol1002005295310
- J.M.McCordOxygen-derived free radicals in postischemic tissue injuryN Eng J Med3121985159163
- J.K.BeckmanT.YoshiokaS.M.KnobelH.L.GreeneBiphasic changes in phospholipid hydroperoxide levels during renal ischemia/reperfusionFree Radic Biol Med111991335340
- N.NitescuS.E.RickstenN.MarcussenB.HaraldssonU.NilssonS.BasuetalN-Acetylcysteine attenuates kidney injury in rats subjected to renal ischaemia-reperfusionNephrol Dial Transplant21200612401247
- D.J.HernandezW.B.RobertsJ.Miles-ThomasA.MagheliS.SahaE.M.SchaefferetalCan ischemic preconditioning ameliorate renal ischemia-reperfusion injury in a single-kidney porcine model?J Endourol22200825312536
- M.O.TimsitR.GadetH.Ben AbdennebiR.CodasP.PetruzzoetalRenal ischemic preconditioning improves recovery of kidney function and decreases alpha-smooth muscle actin expression in a rat modelJ Urol1802008388391
- S.ZhuangB.LuM.PangPostconditioning does not improve renal function or attenuate tubular damage in ischemia/reperfusion-induced acute kidney injury in miceThe Open Pathol J320091821
- Weng X, Shen H, Kuang Y, Liu X, Chen Z, Zhu H, et al. Ischemic postconditioning inhibits the renal fibrosis induced by ischemia-reperfusion injury in rats. Urology 2012 http://dx.doi.org/10.1016/j.urology.2012.02.054.
- L.M.SchwartzC.J.LagranhaIschemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigsAm J Physiol Heart Circ Physiol2902006H10118
- J.DowR.A.KlonerPostconditioning does not reduce myocardial infarct size in an in vivo regional ischemia rodent modelJ Cardiovasc Pharmacol Ther122007153163