Abstract
Objectives:
To report a technique of percutaneous endoscopic nephropexy, using a polyglactin suture passed through the kidney, in patients with nephroptosis.
Patients and methods:
Four women presenting with symptomatic right nephroptosis underwent a percutaneous endoscopic nephropexy. An upper-pole calyx was accessed percutaneously and a 24-F working sheath was placed. Another needle access was made through a lower-pole calyx and a #2 polyglactin suture was passed into the renal pelvis. It was then pulled out through the upper-pole tract using the nephroscope. A retroperitoneoscopy was performed and the tip of the nephroscope was used to cause nephrolysis. After inserting the nephrostomy tube the polyglactin suture was passed into the subcutaneous tissue and then tied without too much tension, to avoid cutting the parenchyma.
Results:
The operative duration was 33 min and the hospital stay after surgery was 3.5 days. The nephrostomy catheter was removed 5 days after surgery. There were no complications, especially no haemorrhagic, infectious, lithiasic or thoracic complications. The four patients were relieved of their initial symptoms, with a mean follow-up of 28 months. Ultrasonography and/or intravenous urography showed the kidney at a higher location with the patient standing.
Conclusions:
This technique combines the nephrostomy tract used in percutaneous techniques with the suture and nephrolysis used in laparoscopic techniques. Moreover, this procedure seems to be safe, with satisfactory anatomical and clinical results and a lower morbidity. However, a larger series will be necessary to establish its long-term morbidity and success rate.
Introduction
Nephroptosis is defined as a renal descent of more than two vertebral bodies when the patient moves from supine to erect (standing). However, the kidney can move back into a normal location when supine, which differentiates it from an ectopic kidney [Citation1]. More than 170 different operative techniques and modifications for nephropexy have been described, but because of their use for the wrong indications, these techniques have been largely discredited [Citation1]. However, in the last decades there has been a renewed interest in symptomatic nephroptosis, with new criteria for diagnosis and new methods of treatment, e.g. laparoscopic and percutaneous nephropexy [Citation1–Citation6]. Here we report a new technique for percutaneous endoscopic nephropexy that combines the nephrostomy tract used in percutaneous techniques, and the suture and nephrolysis used in laparoscopic techniques.
Patients and methods
Four women (mean age 30.5 years, range 24–40) were diagnosed with symptomatic right nephroptosis. They had recurrent right flank or abdominal pain after long periods of standing or increased activity. The patients were typically thin, with a mean (range) body mass index of 19.2 (18.9–20.4) kg/m2. No patient had a high-blood pressure, especially when standing. They had undergone many consultations and several periods of analgesic drug therapy over the previous years, with a mean (range) time to diagnosis of 4 (6–3) years. Ultrasonography showed a descent of the right kidney in the lower abdomen when the patients passed from supine to standing. IVU confirmed the right renal descent, with tilting of the kidney when the patients moved from supine to erect. In two cases, a radionuclide renal scan confirmed the decrease in renal perfusion when the patient moved from supine to standing. The study was approved by the hospital ethics committee. After informed consent, patients underwent a percutaneous endoscopic nephropexy.
Operative technique
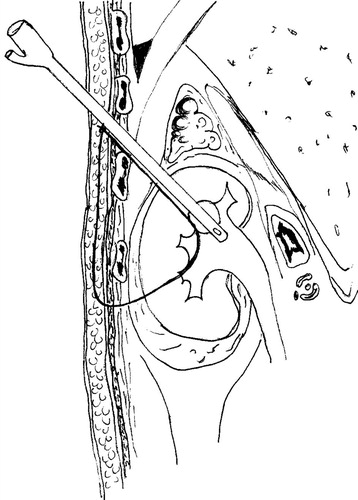
Under general anaesthesia, the patients were placed in a steep head-down split-leg modified lateral position [Citation7], to facilitate the cephalad displacement of the kidney. A 7-F ureteric catheter was inserted to administer contrast medium and to acutely dilate the collecting system. Under fluoroscopic guidance, an upper-pole calyx was accessed via an intercostal route. After inserting a safety guidewire, the tract was dilated, and a 24-F Amplatz sheath was placed. A 20.8-F nephroscope was then positioned in the renal pelvis (). The collecting system was filled once again with contrast material, and another needle access was made through a lower-pole calyx. A 6-F PTFE dilator was placed in the renal pelvis over a guidewire. Subsequently, a #2 polyglactin suture was passed into the renal pelvis through the PTFE dilator, like a guidewire. It was pulled out through the upper-pole tract using the nephroscope (). The Amplatz sheath and nephroscope were retrieved from the percutaneous tract, to outside the kidney and the retro-renal fascia. Then a retroperitoneoscopy was performed, and the tip of the nephroscope was used to achieve a blunt dissection between the retro-renal fascia and the abdominal wall (). The retro-renal fascia was traversed following the guidewire. The dissection was then continued between the kidney and the retro-renal fascia, liberating the posterolateral aspect of the kidney (–). The nephroscope and Amplatz sheath were once again introduced into the calyceal cavity following the security guidewire. A 20-F Foley catheter was positioned in the renal pelvis through the upper tract (). Its balloon was inflated with 3 mL of sterile water and contrast medium. A mild traction was placed on the kidney to bring it to its highest retroperitoneal position, which was confirmed by a nephrostogram. The 18-G diamond-tip needle was passed under the skin, from the upper to the lower puncture site (). The suture was introduced into the needle and pulled out in the upper skin incision (). Thus, the polyglactin suture was passed, into the subcutaneous tissue, from the lower to the upper puncture site, and then tied without too much tension, to avoid cutting the parenchyma ().
Figure 1 An upper-pole calyx is accessed via an intercostal route; the nephroscope is positioned in the renal pelvis. Another needle access was made through a lower-pole calyx, then a hydrophilic guidewire, and a 5- or 6-F PTFE dilator were inserted. Alternatively, the lower-pole calyx puncture could be made at the start, before upper-pole access.
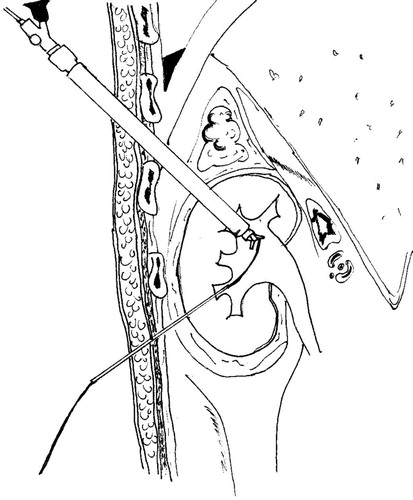
Figure 2 A nephroscopic view; a two-prong forceps grasps the #2 polyglactin suture, which is passed into the renal pelvis via a 6-F PTFE dilator. The suture is pulled out through the upper-pole tract using the nephroscope.
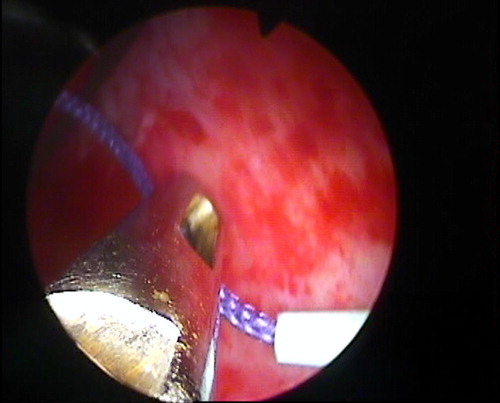
Figure 3 (A) A retroperitoneoscopic view, after the Amplatz sheath and nephroscope were withdrawn in the percutaneous tract outside the retro-renal fascia. A space is developed outside the retro-renal fascia. The black hydrophilic guidewire and the purple suture are traversing the percutaneous tract gap. (B) The retro-renal fascia was traversed following the guidewire. A nephrolysis is developed, liberating the posterolateral aspect of the kidney, until reaching the lower pole access with the purple suture penetrating the renal capsule and parenchyma. (C) Sometimes a blunt dissection is performed with the help of the ‘opening and closing’ of a two-prong forceps.
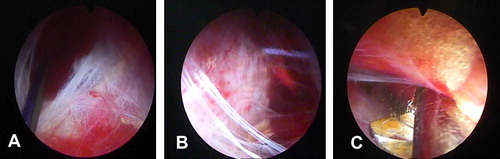
Figure 4 The balloon of the nephrostomy Foley catheter was inflated with 3 mL of sterile water and contrast medium. A mild traction was placed on the kidney to bring it against the abdominal wall. The #2 polyglactin suture is passed into the renal collecting system between the lower and the upper calyx, in the subcutaneous tissue between the upper and lower skin punctures, and then tied in the upper skin incision.
Results
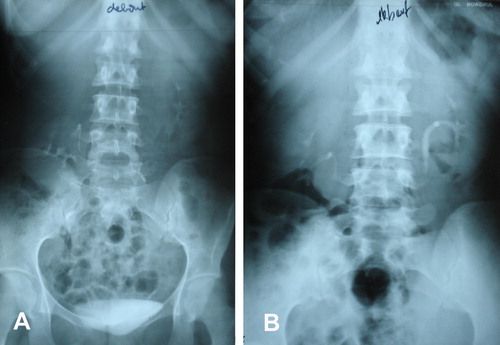
Percutaneous nephropexy was technically possible in all four patients, and the mean (range) operative duration was 33 (28–37) min. Analgesic intravenous paracetamol was used on the first day after surgery, and no further analgesic therapy was needed. To allow initial scarring and fixation of the kidney, the patients were advised to stay in bed in the dorsal position for 2 days. Mechanical methods of thromboprophylaxis (anti-embolism stockings, leg movements), and low-molecular-weight heparin, were used to prevent thromboembolic complications. One patient had a mild fever (38.7 °C) on the first day, but that resolved spontaneously. Although there was a retroperitoneal dissection there was no large extravasation, nor any other complication, especially haemorrhagic, infectious or thoracic. The patients had an uneventful discharge and the mean (range) hospital stay after surgery was 3.5 (2–5) days. The nephrostomy catheter was removed 5 days after taking the nephrostogram. There were no late complications secondary to the suture into the calyceal system, and no haemorrhage, infection or stone formation. The patients reported a subjective resolution of their initial symptoms. Ultrasonography and/or IVU showed the kidney at a higher position, with the patient erect, than before surgery (), with a mean (range) follow-up of 28 (7–60) months.
Discussion
Currently, advanced radiological imaging has allowed the accurate diagnosis of well-documented symptomatic nephroptosis. Moreover, the advent of minimally invasive surgical techniques, which have lower morbidity, has restored interest in nephroptosis, and generated reports of new operative techniques in its management [Citation1].
In the present study we showed the feasibility and describe in detail a new technique of percutaneous nephropexy. Our technique combines some features of percutaneous and laparoscopic nephropexy. Thus, it used a nephrostomy tract, a percutaneous suture, and nephrolysis through the unique percutaneous tract. In these few patients the procedure was possible in a quick operation, and with little or no morbidity. In addition, it was effective, as the patients reported a subjective resolution of the symptoms during the follow-up.
Laparoscopic nephropexy is considered the standard therapy for symptomatic nephroptosis [Citation1], as it provides a successful outcome in 85–100% of patients [Citation6]. Moreover, it causes less postoperative pain, a briefer hospitalisation, lower morbidity, and briefer convalescence than after open surgery [Citation1].
Several methods of percutaneous nephropexy have been reported, with an ≈88% success rate as assessed symptomatically [Citation1,Citation2,Citation4]. Moreover, they are a good option in centres with little or no access to laparoscopic expertise or equipment [Citation1]. Thus, a ‘one-point nephropexy’ was reported using a simple nephrostomy tube placement [Citation2], or a nephropelvi-ureteric catheter inserted percutaneously [Citation3]. For more anchoring, a two-point technique was used with a circle (U) nephrostomy tube [Citation4] or with two Council-tip catheter nephrostomy tubes [Citation5].
The rationale for these percutaneous nephropexy methods is the observation that after placing a percutaneous nephrostomy tube, the scar is sufficient to hold the kidney in place [Citation1,Citation2,Citation4]. However, compared to laparoscopy, the main limitation of percutaneous nephropexies is the inability to perform an adequate nephrolysis, to induce adhesions and form a ‘surgical scar’ to secure the kidney firmly in a higher position [Citation1]. Consequently, a perirenal dissection, as in our technique, is used to induce these additional adhesions, which might be more resistant to gravity than the nephrostomy tract alone. In addition, the polyglactin suture holds the kidney in place, giving time for perirenal fibrosis to organise and mature. Therefore there is no need for prolonged indwelling of a nephrostomy tube. Moreover, our procedure has the advantages of percutaneous nephropexy, i.e. it has low morbidity, is easy to perform, is low-cost, takes less time and is as effective as laparoscopy. Also, it has the advantages of laparo-endoscopic single-site surgery, e.g. reduced morbidity and improved cosmetic results [Citation8].
However, this technique has the disadvantages of percutaneous renal surgery, i.e. a risk of haemorrhage and risks of intercostal tract complications. Also, with the polyglactin suture through the collecting system, there was concern about percutaneous fistulation, tearing through the renal parenchyma, and the risk of stone formation, none of which occurred in any patient. This technique cannot detect nor correct an incomplete colon rotation, which is a possible underlying pathogenic mechanism of nephroptosis [Citation9]. Also, the study has some limitations, as there were too few patients to draw firm conclusions other than the feasibility of the procedure. There was no objective evaluation of the patients (quality-of-life questionnaires, pain analogue scales).
For future improvement and less invasiveness it would be possible to perform a renal dilatation of 10 F, and use a rigid ureteroscope to extract the suture, without a working sheath, similar to the technique we use to make a second simultaneous percutaneous tract in complex stone extraction [Citation10]. The nephrolysis might be performed with the same rigid ureteroscope or with the nephroscope after dilating the percutaneous entry alone.
In conclusion, this technique of percutaneous nephropexy appears to be a feasible treatment option for patients with symptomatic documented nephroptosis. However, a longer follow-up and more extensive experience, with an objective evaluation before and after surgery, are needed to verify the safety and effectiveness of this technique.
Conflict of interest
None.
Funding
The study and the report were not funded.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- S.J.SrirangamA.J.PollardA.A.AdeyojuP.H.O’ReillyNephroptosis: seriously misunderstood?BJU Int1032009296
- A.M.KhanE.HolmanC.TothPercutaneous nephropexyScand J Urol Nephrol342000157161
- M.Castillo RodríguezE.Larrea MasvidalD.Hernández SilverioA.Carauna Valdes-GómezM.V.Labrada RodríguezT.Cuesta MegiasPercutaneous nephropexy in the treatment of renal ptosisArch Esp Urol521999250256
- J.G.SzékelyRe. Laparoscopic nephropexy: Washington University experience (letter to the editor)J Urol1571997266
- D.M.HoenigA.K.HemalA.L.ShalhavR.V.ClaymanPercutaneous nephrostolithotomy, endopyelotomy and nephropexy in a single sessionJ Urol1601998826827
- J.T.BishoffL.R.KavoussiLaparoscopic surgery of the kidneyP.C.WalshA.B.RetikE.D.VaughanA.J.WeinCampbell’s urology9th ed.2007SaundersPhiladelphia17591809 [Chapter 51]
- M.LezrekA.AmmaniK.BazineM.AssebaneH.Kasmaoui elA.Qarroet alThe split leg modified lateral position for percutaneous renal surgery and optimal retrograde access to the upper urinary tractUrology782011217220
- E.LiatsikosI.KyriazisP.KallidonisM.DoA.DietelJ.U.StolzenburgPure single-port laparoscopic surgery or mix of techniques?World J Urol302012581587
- J.WadstromM.HaggmanLaparoscopic nephropexy exposes a possible underlying pathogenic mechanism and allows successful treatment with tissue gluing of the kidney and fixation of the colon to the lateral abdominal wallInt Braz J Urol3620101017
- M.LezrekK.H.BazineA.AmaniM.AssebaneO.GhoundaleA.Qarroet alV30 ‘tips and tricks’ of percutaneous surgery in the split leg modified lateral position: optimal simultaneous anterograde and retrograde accessEur Urol Suppl102011351