Abstract
Penile vascular surgery for treating erectile dysfunction (ED) is still regarded cautiously. Thus we reviewed relevant publications from the last decade, summarising evidence-based reports consistent with the pessimistic consensus and, by contrast, the optimistically viable options for vascular reconstruction for ED published after 2003. Recent studies support a revised model of the tunica albuginea of the corpora cavernosa as a bi-layered structure with a 360° complete inner circular layer and a 300° incomplete outer longitudinal coat. Additional studies show a more sophisticated venous drainage system than previously understood, and most significantly, that the emissary veins can be easily occluded by the shearing action elicited by the inner and outer layers of the tunica albuginea. Pascal’s law has been shown to be a significant, if not the major, factor in erectile mechanics, with recent haemodynamic studies on fresh and defrosted human cadavers showing rigid erections despite the lack of endothelial activity. Reports on revascularisation surgery support its utility in treating arterial trauma in young males, and with localised arterial occlusive disease in the older man. Penile venous stripping surgery has been shown to be beneficial in correcting veno-occlusive dysfunction, with outstanding results. The traditional complications of irreversible penile numbness and deformity have been virtually eliminated, with the venous ligation technique superseding venous cautery. Penile vascular reconstructive surgery is viable if, and only if, the surgical handling is appropriate using a sound method. It should be a promising option in the near future.
Abbreviations:
- CC, corpora cavernosa
- ED, erectile dysfunction
- DDV, deep dorsal vein
- CV, cavernous vein
- PAV, para-arterial vein
- ERV, erection-related vein
- VOD, veno-occlusive dysfunction
- PRS, penile revascularisation surgery
- IEGA, inferior epigastric artery
- PCL, penile crural ligation
- DPVL, dorsal penile vein ligation
- PVS, penile venous stripping
Introduction
The human penis has been in its current anatomical form for the last 2000 centuries [Citation1] and despite extensive studies, it is likely that its anatomy and the erection process are still not thoroughly understood [Citation2]. Humans are peculiar within the group of erect animals, in that the males possess an os analogue associated with a proportionally large and extraordinarily extensible corpora cavernosa (CC), but man does not have an os penis, which is present in all quadrupeds [Citation3], i.e. the bony portion that provides penile rigidity. The erectile capability of the human penis largely depends on sinusoids in the glans penis, the corpus spongiosum and the CC, which are also exclusively responsible for erectile rigidity [Citation4]. Therefore it seems that the human CC are destined to be prone to erectile dysfunction (ED), defined as inability either to attain or maintain a rigid erection for satisfactory intercourse [Citation5].
A new understanding of penile anatomy and the erection process
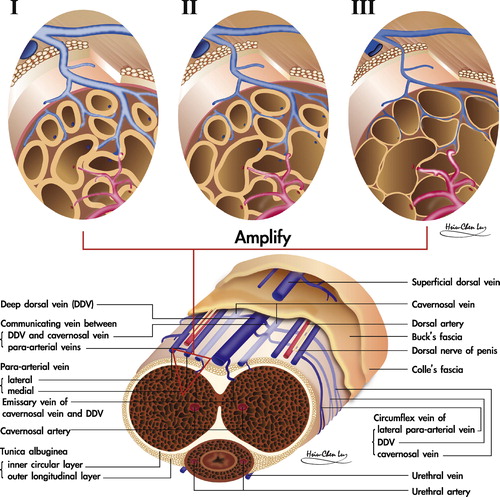
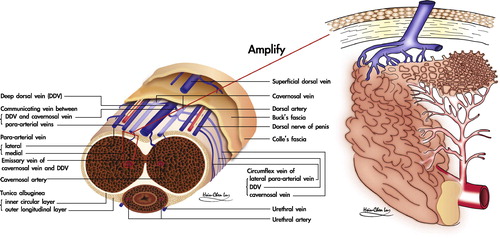
Recent studies substantiate a model of the tunica albuginea of the CC as a bi-layered structure with a 360° complete inner circular layer and a 300° incomplete outer longitudinal coat spanning from the bulbospongiosus and ischiocavernosus proximally, and extending continuously into the distal ligament within the glans penis [Citation6]. The entire outer layer of the penis and the above two muscles could be collectively categorised as skeletal components. The inner layer contains and supports the intracavernous sinusoids, the erection-related veins and artery which could collectively be allocated as the smooth muscle components [Citation7].
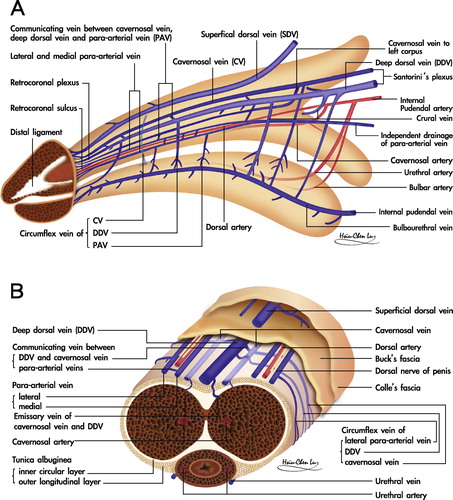
A rigid erection of the CC is initiated by either central or local sexual stimuli, if there are healthy CC in which a unique dual circulatory route is characteristic [Citation8]. Apart from the regular vascular system for nutrition via the capillaries, there is a system for erectile function in which sinusoids shunt directly from arterial to venous channels bypassing the capillaries. In the human penis, the vascular distribution is of a particular design (). The main source of blood supply to the penis is from the internal pudendal artery, which is the end branch of the internal iliac artery, although accessory contributions might arise from the external iliac, obturator, vesical and femoral arteries. The internal pudendal artery becomes the common penile artery after giving off a branch in the perineum.
Figure 1 A schematic illustration showing the vascular system in the human penis. (A) Upper lateral view. The DDV is consistently in the median position, receives the blood of the emissary veins from the CC and of the circumflex vein from the corpus spongiosum. It is sandwiched by CVs, although these lie in a deeper position. Bilaterally each dorsal artery is sandwiched by its corresponding medial and lateral PAVs, respectively. Note that the lateral PAV merges with the medial one proximally. The deeper colour of the veins indicates the deepest group of the vasculature. The internal pudendal artery gives a branch to bulbourethral artery and then the cavernous artery, which is the major supplier of the CC as well as the dorsal artery supplying the glans penis. (B) A cross-section of the mid-portion. Note that there are seven veins rather than the traditionally reported one, although it becomes four at the level of penile hilum because a merger in each pair of the nomenclature veins. The ERVs are arrayed in an imaginary arc on the dorsal aspect of the tunica albuginea. The penile artery does not always accompany fellow veins.
The sinusoids of the CC are primarily supplied by the cavernous artery, which is the second last branch of the internal pudendal artery. The internal pudendal artery also gives rise to the bulbourethral branches proximally, and distally the dorsal artery, which supplies the glans and the CC.
The sinusoidal blood drains directly to the subtunical venous plexus, subsequently passing through the tunica albuginea to the emissary veins, and then to the deep dorsal veins (DDVs), cavernous veins (CVs) and para-arterial veins (PAVs). DDVs, CVs and PAVs are collectively termed erection-related veins (ERVs) because they drain the sinusoidal blood separately [Citation9]. This new understanding of penile venous anatomy is highly significant. Not only is the traditional DDV considered significant, but also the CV (which distributes through the entire penile length) and the PAVs. The DDV that lies consistently in the median position receives blood from the circumflex veins of the corpus spongiosum and from the emissary veins of the CC. Emissary veins () lie between the inner and outer layers of the tunica for a short distance, often piercing the outer layer bundles obliquely. Therefore, and significantly, these emissary veins can be easily occluded by the shearing action caused by the inner circular and outer longitudinal layers of the tunica albuginea.
Figure 2 An illustration showing the penile erection process. The lower panel shows a cross section of the human CC. In the upper panel, from left to right, the sinusoids of the CC expand gradually when the helicine artery dilates to increase the blood flow, as the outer longitudinal layer of the tunica albuginea limits and eventually seals off the emissary vein, resulting in penile erection.
The DDV is sandwiched by cavernosal veins that are coalesced to one at the penile base. Bilaterally each dorsal artery is sandwiched by the medial and lateral PAV, respectively. Veins from the glans penis form a retrocoronal plexus that drains predominantly into the DDV, the urethral veins and the corpus spongiosum. The DDV courses proximally along the midline above the CC and empties into the periprostatic plexus [Citation10]. The superficial dorsal vein drains the skin and the subcutaneous tissue superficial to Buck’s fascia, and might have direct shunts to the CC. This in turn drains into the superficial external pudendal vein. If, and only if, the erectile function of the CC is healthy, the penis not only expands in its girth, but also increases in length, resulting in sufficient rigidity with sexual arousal.
Two types of branches arise from the cavernous artery. First, the outer capillaries that are responsible for penile nutrition during the flaccid state, and supply the smooth muscle and nerve fibres. This system signifies that the CC is also susceptible to long-hours in the erectile state. Second, and for fulfilling erection function, the inner helicine arteries [Citation11] open directly into cavernous spaces without entering capillaries (), which are then emptied into the post-cavernous venules. These inner arteries are shaped-like corkscrews and allow the penis to elongate and dilate without compromising flow. Multiple layers of smooth muscle surround the helicine branches. This muscle is contracted while flaccid, allowing only small amounts of blood into the lacunar spaces. After the appropriate stimulus, muscle relaxation occurs and the arteries dilate and straighten, increasing blood flow and dilating the lacunar spaces, in turn, applying pressure against the outer longitudinal layer of the tunica albuginea to reduce the blood drainage, which eventually results in a rigid erection. Application of real world physics would assume that the CC is an ideal vessel to apply Pascal’s Law which states that pressure applied to any part of an enclosed fluid at rest is transmitted undiminished to all walls of the containing vessel [Citation12].
The pathophysiology of ED
What leads to an adequate erection is probably still not completely explored, with the current consensus remaining multifaceted and complex [Citation13]. There must be freedom from psychological disturbance, an intact neurological system, a normal hormonal profile, no adverse drug influence, no systemic diseases, a functional artery and healthy intracavernous tissues [Citation14]. End-organ function requires arterial sufficiency, normal sinusoidal tissue and functional veins. Although there is no agreement on what would be the major contributor, there is a bias towards endothelial function, because of the dramatic effects of phosphodiesterase-5-inhibitors on ED.
However, contrary to this are recent haemodynamic studies on fresh and defrosted human cadavers, whereby rigid erections were unexceptionally reproduced despite the lack of endothelial activity [Citation15]. Constant low-pressure perfusion was used to mimic arterial inflow, and the staged removal of ERVs produced increasingly more rigid erections. Could it be that ERVs might in fact be the predominant factor that underlies erectile rigidity? Certainly, and in the light of the increased understanding of the penile vasculature, it would at least warrant a re-evaluation of the role of venous surgery for ED [Citation16].
Briefly, the CC and skeletal muscle components unite with smooth muscle components to meet the requirements for erection, and only allow vascular and nervous tissue to communicate with the systemic circulation. Their anatomical relationship is apparently like a cluster of grapes, when the emissary vein is regarded as the grape trunk and each sinusoid as a fruit (). The rigid erection of the CC overall depends on cooperation among healthy sinusoids, a normal tunica, functional arteries, and competent veins. Thereafter, vascular dysfunction is an important cause of male ED, and can be classified as veno-occlusive dysfunction (VOD), arterial insufficiency, or mixed. Accordingly, the best resort might be penile venous surgery, arterial reconstruction and venous/arterial reconstruction, respectively.
Figure 3 An illustration of the relationship between the CC sinusoids and emissary vein. Left: A cross section of the human CC. Right: An amplified view of an emissary vein and its supplying sinusoids. Their anatomical relation is like a cluster of grapes, when the emissary vein is regarded as the grape trunk and each sinusoid as a fruit.
A review of arterial revascularisation/veno-arterialisation techniques for restoring erectile function
Arterial vasculogenic causes of ED increase with age and are most prevalent in those with risk factors for atherosclerosis (obesity, smoking, hypertension, diabetes and hyperlipidaemia). Traumatic events, which occur more often in younger men, are due to pelvic and/or perineal trauma. Arterial insufficiency can be detected using colour Doppler ultrasonography, if the peak systolic velocity is less than 25 cm/s. Angiography, whilst being the benchmark for all arterial investigations, is reserved with the aim of concurrent embolisation or angioplasty if an arteriovenous fistula or stenosis is suspected.
Penile revascularisation surgery (PRS)
In 1973, Michal et al. [Citation17] reported the first study of penile arterial reconstruction in an attempt to enhance arterial flow to the CC. They devised PRS using the anastomosis of the inferior epigastric artery (IEGA) to the CC. Subsequently, DDV arterialisations, either with no extensive venous ligation or with venous ligation, were introduced by Virag [Citation18] and Haudi [Citation19] in 1981 and 1986, respectively. In an effort to deliver better outcomes, various modifications were used, such as the Furlow–Fisher procedure (), in which IEGA was made in an end-to-side anastomosis to the DDV, while several ligations were made at the lateral circumflex veins, in proximal and distal locations [Citation20]. The outcomes of arterial PRS are varied due to many causes, e.g., patient selection, surgical instruments and surgical technique. In general, its merit is limited, with no long-term benefits (). Significant complications appear to be unavoidable, i.e., the risk of postoperative penile shortening and decreased penile sensation. Not surprisingly, it has been regarded as experimental among most surgeons [Citation21].
Figure 4 An illustration of the Furlow–Fisher procedure. The inferior vein is fashioned to the DDV, which is ligated distally, proximally and at lateral locations of the circumflex veins.
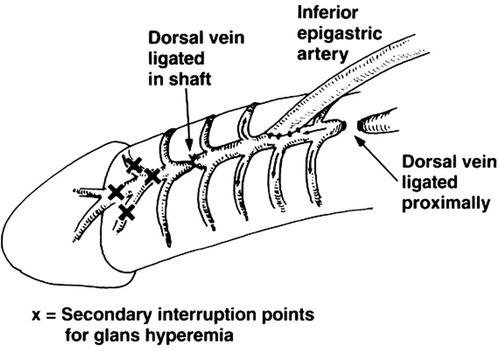
Table 1 The outcomes from PRS (adapted from [Citation26]).
In 1996, a high point was reached when the AUA guidelines [Citation22] considered PRS and penile venous surgery in men with atherosclerotic disease as investigational, and recommended that it should be used only in a research setting with a long-term follow-up available. The proceedings of the First Paris International Consultation on Erectile Dysfunction in 1999 [Citation23], the second in 2003 [Citation24] and the third in 2009, were in agreement with the AUA guidelines. Similarly, in 2005, the AUA Update on the Management of ED [Citation25], and the International Society for Sexual Medicine textbook, Standard Practice in Sexual Medicine [Citation26] maintained the consensus. However, it might deserve reappraisal after a review of arterial reconstructions reported after 2004 () [Citation27–Citation32], Interestingly, researchers unexceptionally report positive long-term outcomes when both penile arterial and venous reconstruction are incorporated, suggesting that arterial reconstruction alone is insufficient for the long-term restoration of erectile function. For investigating the vascular contribution exclusively, haemodynamic studies have been used in both fresh and defrosted human male cadavers since 2001 [Citation33]. In each case, a rigid erection was attainable at a very low infusion rate, after venous removal. This clearly has significant ramifications in relation to vascular reconstruction and its role in treating patients with ED. Overall successful revascularisations are prominent in young patients who have sustained focal arterial injury due to trauma, and in older healthy men with recently acquired ED secondary to a focal arterial occlusion, and in the absence of any evidence of generalised vascular disease [Citation33].
Table 2 Outcomes from PRS in recent decades.
Surgery for correcting VOD
In 1973, Parona [Citation34] hypothesised that the penile dorsal vein might be dysfunctional as a result of varicosity. In 1902, Wooten [Citation35] made the first dorsal penile vein ligation (DPVL) for atonic impotence. Although Lowsley and Rueda [Citation36] reported this procedure in more men in 1953, DPVL for restoring erectile function had not been popular until 1985 [Citation37]. The approach was expanded from initial procedures involving single-vessel ligation of the DDV to more elaborate techniques in which excision of the DDV, CV, and the crural vein were described [Citation38]. It appears that the offending veins of VOD are merely a single DDV with tributaries, crural veins and CVs that merely drain the penile hilum. Not surprisingly, DPVL has been almost abandoned because the general consensus for this type of treatment for ED is a short-term success of 1–2 years, with no sustainable long-term outcome [Citation39]. In addition to the lack of sustained functional improvement, irreversible deformity and permanent numbness of the penis after surgery were frequent, and considered to be unacceptable complications [Citation40]. Failure to achieve a long-term solution for ED and the seemingly unavoidable adverse side-effects have discouraged many surgeons from using DPVL for patients with ED. However, irreversible numbness results from nerve damage, and penile deformity is a result of either fibro-skeletal alteration, or loss of extensibility and the sliding capability of layered tissues from electrocoagulation-induced fibrosis [Citation41]. So should neither nervous nor fibrous tissues be unaffected if DPVL is exclusively targeted to venous structures? Should the venous removal be sufficient if all conventional penile venous anatomy has exclusively depicted a single DDV and tributary veins near the penile hilum only?
Positive reports of venous reconstruction are limited to penile crural ligation (PCL) [Citation42], apart from those of Hsu et al., whose method has addressed penile venous stripping (PVS). Again, authors seem to recommend PCL, which enhances penile venous impedance. In the long term, a collateral circulation will probably ensue, as the entire CC can be regarded as an enclosed chamber. Why will penile venous stripping not be effective if PCL works? Despite the review of all related publications over the last decade, PVS is still regarded as experimental. The advances in the understanding of penile anatomy, in surgical instruments and surgical techniques have resulted in us using ambulatory PVS on >3000 men since 1986.
The chronological development of a refined method of penile venous stripping
After microsurgery training on rat and human cadaveric dissections of the entire penile structure in 1985, neither electrosurgery nor a suction apparatus has been used with our penile venous stripping techniques (). In 1986 we introduced DPVL in an initial study of eight men [Citation43]. This simple procedure was replaced by venectomy soon thereafter, and then by PVS, which was based on the conventional penile venous anatomy of a single DDV until 1999. The rate of successful intercourse has improved from 50% to 91% in the past decade.
During 1999, 35 patients had repeat cavernosography because of a gradual decrease in their erectile capability over a period of 6 months to 7 years after surgery [Citation44]. Imaging showed some excessive veins (CV and PAV) that were further confirmed in cadaveric dissections. By then, the revolutionary view of erection-related veins in the human penis () was regarded as a blueprint for PVS [Citation45]. The latest method of PVS is based on a circumferential incision plus a median pubic longitudinal approach via a specific key instrument, with an acupuncture-assisted local anaesthesia on an ambulatory basis [Citation46]. As the PVS is exclusively directed at the veins, damage to non-venous soft tissues is avoided. Tissues like nerve, artery and lymphatic vessels are spared. Since June 1988 we have used the PVS procedure as outpatient surgery under local anaesthesia. Neither electrosurgery nor a suction apparatus has been required in the entire procedure, resulting in minimal or no injury to any tissue other than the veins. The incision is small and delicate. Contrary to the feared potential recurrence, <25% of the 3000 patients who had the operation have complained of a recurrence. Unfortunately, the overall outcomes are too difficult to report in one article, due to the series of methods developed over several decades. provides a comprehensive and chronological review [Citation9,Citation16,Citation43,Citation45,Citation47–Citation49]. Although the use of electrocautery is routine in surgery, it is a major avoidable hazard in PVS [Citation47].
Figure 5 Anatomy-based penile venous stripping, shown chronologically. Upper panel: The conventional penile venous anatomy, of a single DDV, had provided the surgical blueprint from 1986 to 1999. Middle panel: From 1999 to 2003, a CV was identified because it was distributed in the entire CC, (Reproduce in part from [Citation45], with permission). Lower panel: A DDV, two CVs and two pairs of PAVs were recognised after 2004 (Reproduced with permission from [Citation44]).
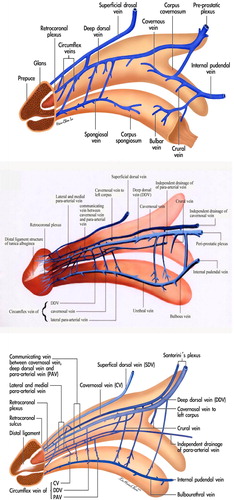
Table 3 Methods of penile venous surgery developed chronologically since 1986.
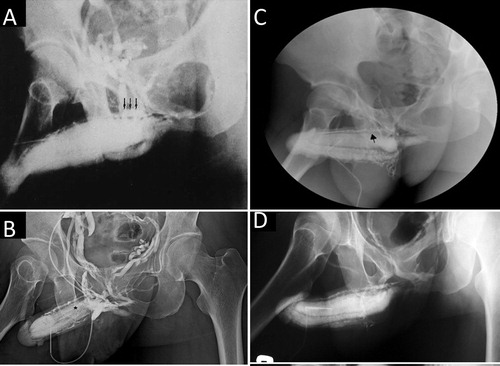
The second reason for unsuccessful conventional PVS is the incomplete removal of offending veins for VOD if the PVS refers to the conventional penile venous anatomy [Citation48]. Both residual veins and electrocautery effects are readily detected in those patients who underwent PVS somewhere internationally in 2008 ().
Figure 6 Inadequate penile venous surgery. (A) Residual veins (arrow) were significant. (Reproduced with permission from [Citation48]). (B) Not only were the residual veins significant, but there was also poor intercavernous filling with contrast medium in the pendulous portion of the CC, implying an adverse effect of the electrocautery. (C) A ligation (arrow) was noted proximally while there was also poorer sinusoidal filling. (D) The entire venous system remained untouched.
The latest refined penile venous stripping
In medical history it is rare to encounter a surgical procedure like venous surgery for restoring erectile function that has sustained such an extended period of disrepute. For over a century, the merit for conducting penile venous surgery to treat ED has never been firmly established. It was believed to be indicated only for <1% of patients with ED secondary to VOD. However, recent studies show that VOD might be more prevalent in patients with ED, and even in those with ED ascribed to penile arterial insufficiency [Citation50]. Thus, the prevalence of VOD could be greater than expected, suggesting that a larger proportion of patients with ED might be suitable for venous surgery.
Acupuncture-assisted local anaesthesia and documenting VOD
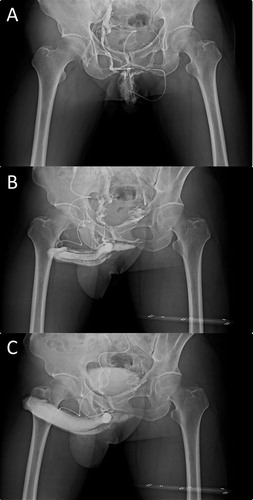
A diagnosis of VOD was made based on dual pharmaco-cavernosography and Doppler ultrasonography. These cavernosographic studies () consisted of injecting 120 mL of a 30% contrast solution intracavernously via a 19-G scalp needle firmly affixed to the lateral distal penis. The first set of cavernosograms was taken to show the penile venous anatomy. The second set was made within 30 min after an injection with 20 μg prostaglandin E1 via the same intracavernous route, to document VOD if venous channels were shown during a rigid erection after the infusion of contrast solution (and, if necessary, additional saline). The arterial pulsatile function and the response to prostaglandin E1 were simultaneously assessed. Doppler ultrasonography of the penis was used during a separate visit to exclude arterial insufficiency.
Figure 7 Dual cavernosography. (A) A cavernosogram showed the one independent DDV and a pair of CVs. (B) A late phase of the film showed the complex venous system. (C) A pharmaco-cavernosogram showed VOD, because of the significant veins despite a rigid erection ensuing.
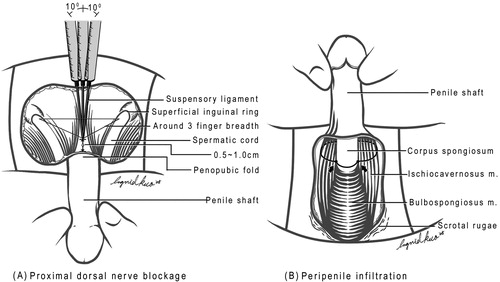
On applying a proximal dorsal nerve blockage (), lidocaine (0.8%, 50 mL) with adrenaline was delivered between the suspensory ligament along the symphysis pubis using a 23-G disposable needle with the bevel parallel to the longitudinal body axis. The penile shaft was pulled away from the body axis, while the solution was injected via a 10-mL syringe into the penile hilum and into the bilateral proximal dorsal nerves. Scrotal and topical infiltrations of the injected lidocaine at the junction between the corpus spongiosum and corpora cavernosa were then made using finger-guided manipulation. The dosage of lidocaine was limited to 400 mg. Acupuncture was used as an adjuvant to local anaesthesia, if a patient became tense during cavernosography/ultrasonography or felt uneasy with skin scrubbing from the preoperative preparations. Those patients were selected in view of their intolerance to light pain. The acupoints of Hegu, Shou San Li, Quchi, and Waiguan were routinely stimulated. The Hegu point is located at the highest point of the prominence when the thumb and the index finger are kept adducted. The Quchi point is at the lateral end of the ‘transverse cubital crease’, with the elbow flexed at a right angle. The Shou San Li point is positioned 3 finger breadths caudally to the Quchi acupoint. The Waiguan point is two finger breadths proximal to the middle point of the volar transverse carpal crease, between the flexor carpi radialis muscle and the palmaris longus tendon, with the forearm prone.
Figure 8 An illustration of the method of local anaesthesia. (A) The proximal dorsal nerve block; a 10-mL syringe with a 23-G (3.18 cm) disposable needle was used to inject a local anaesthetic of 0.8% 50-mL lidocaine solution. (B) A peri-penile infiltration was subsequently applied with finger-guided manipulation. (Reproduced with permission from [Citation50]).
Penile venous stripping surgery
A circumferential incision was made first, while a circumcision was completed if required (). The tissue layers superficial to the Colles’ fascia were then more extensively degloved. A ‘milking’ manipulation is helpful to enhance the visibility of the DDV, which is stripped proximally with a pull-through technique via an opening made on Buck’s fascia, from opening to opening on Buck’s fascia up to the penile base, using a 6–0 Nylon suture. Likewise, the paired CVs were stripped. For accessing the deep-seated venous vessels, a median longitudinal pubic incision was used to continue the venous stripping.
Figure 9 Photographs of venous stripping surgery. (A) A circumferential incision was made, followed by a ‘milking’ manipulation (squeezing the sinusoids and venous plexus) to enhance the visibility of the veins. (B) The trunk of the DDV was freed over a 1-cm segment and was then held distally and proximally with a haemostat, after which it was cut. (C) The proximal stump serves as a guide for performing the pull-through manoeuvre up to the penile base. (D) The CVs were managed similarly. (E) A median longitudinal pubic wound was made to relay the venous stripping. (F) The DDV was managed in the deep position. (G) The CVs were manipulated similarly. (H) Both circumferential and pubic wounds were closed.
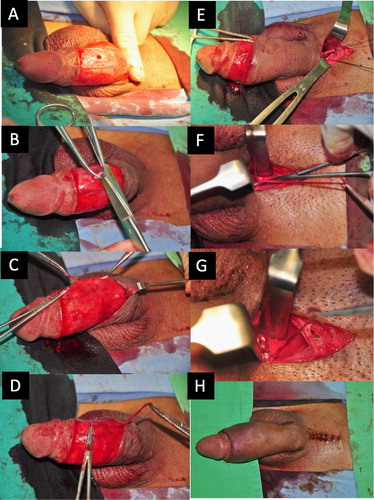
The paired proximal stumps of the DDV and the CVs served as a guide, and the DDV was then firstly thoroughly stripped and ligated using an 85° haemostat as far as the pubic angle. Similarly, the deep-seated CVs are managed. The paired PAVs that sandwich the dorsal arteries bilaterally were meticulously ligated rather than stripped. Smaller veins between the tunica albuginea and Buck’s fascia were identified by squeezing the sinusoids of the CC. The wound was closed with 5–0 chromic catgut and 6–0 Nylon sutures. A compression dressing was placed such that it encircled the penile shaft. Follow-up cavernosograms showed the ideal milieu of the CC for retaining intracorporeal fluid/blood (). These contingent techniques have been applied since 1986, along with many minor modifications since that time.
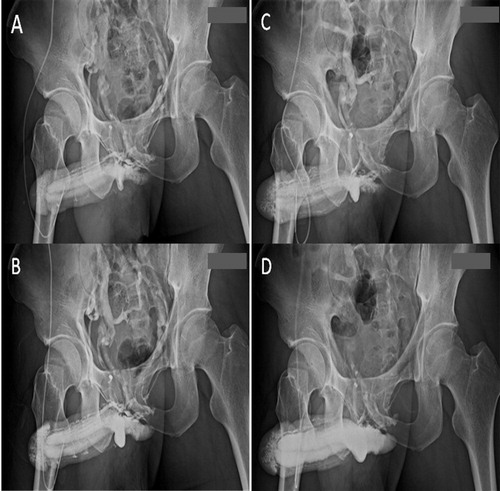
Figure 10 A cavernosographic indicator for assessing surgery. (A) A preoperative cavernosogram shows the venous system in an early phase. (B) There were more veins apparent in a later phase. (C) The postoperative film showed fewer veins, while the penile crura were readily filled. This provides a comparison with the view in panel A. (D) A late-phase film for comparison with that in panel B. Overall these films provide an objective indication for assessing the efficacy of PVS.
The future direction of PVS
In the past three decades there has been a rapid increase in new technologies, devices, gadgets and the application of state-of-the-art tools to medicine and surgery. PVS has benefited significantly from this trend. It should be a promising method in the future if, and only if, a sound method is used and with valid instruments.
Summary
In recent decades there have been many new insights into penile tunical and venous anatomy, and in erectile physiology. These insights have underpinned penile morphological reconstructive, and penile vascular surgery. Although vascular reconstructive surgery is still regarded as experimental, anatomically based techniques of vascular reconstruction should be re-appraised. Arterial reconstruction has been shown to be beneficial in young healthy men who sustain trauma. Older healthy men with ED are more likely to benefit if the vascular surgery includes both arterial revascularisation and veno-arterilisation. The reproducible refined penile venous stripping procedure, that has been developed and refined over several decades, is showing promise as a viable solution for VOD in men of all ages.
Conflict of interest
None.
Source of funding
None.
Acknowledgements
We thank Ms Hsiu-Chen Lu for illustrations, Mrs Yi-Ting Wang, Luan-Hua Ho, Nicola Chen and Yu-Jen Lin for their preparations of photographs for this paper.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- Fossil reanalysis pushes back origin of Homo sapiens. Scientific American: February 17, 2005.
- C.GratzkeJ.AnguloK.ChitaleyY.T.DaiN.N.KimJ.S.Paicket alAnatomy, physiology, and pathophysiology of erectile dysfunctionJ Sex Med72010445475
- G.L.HsuC.W.LinC.H.HsiehJ.T.HsiehS.C.ChenT.F.Kuoet alDistal ligament in human glans. A comparative study of penile architectureJ Androl262005624628
- R.C.DeanT.F.LuePhysiology of penile erection and pathophysiology of erectile dysfunctionUrol Clin North Am322005379395
- J.KaminetskyEpidemiology and pathophysiology of male sexual dysfunctionInt J Impot Res202008310 S3–S10
- G.L.HsuG.B.BrockL.Martinez-PineiroL.NunesB.von HeydenT.F.LueThe three-dimensional structure of the tunica albuginea: anatomical and ultra-structural levelsInt J Impot Res41992117129
- G.L.HsuC.H.HsiehH.S.WenW.L.HsuC.W.ChenAnatomy of the human penis: the relationship of the architecture between skeletal and smooth musclesJ Androl252004426431
- Y.BanyaT.UshikiH.TakaganeH.AokiT.KuboT.OhhoriTwo circulatory routes within the human corpus cavernosum penis. A scanning electron microscopic study of corrosion castsJ Urol1421989879883
- G.L.HsuC.H.HsiehH.S.WenY.C.ChenS.C.ChenM.S.MokPenile venous anatomy. An additional description and its clinical implicationJ Androl242003921927
- T.F.LueVeno-occlusive dysfunction of corpora cavernosa: comparison of diagnostic methodsJ Urol1551996786787
- F.MontorsiM.SarteschiT.MagaG.GuazzoniG.F.FabrisP.RigattiFunctional anatomy of cavernous helicine arterioles in potent subjectsJ Urol1591998808810
- D.HallidayPascal’s principle, fluidsD.HallidayR.ResnickJ.WalkerFundamentals of physics1997J. WileyNew York355356
- I.Sáenz de TejadaJ.AnguloS.CellekN.González-CadavidJ.HeatonR.Pickardet alPhysiology of erectile functionJ Sex Med12004254265
- G.StefanoR.KreamReciprocal regulation of cellular nitric oxide formation by nitric oxide synthase and nitrite reductasesMed Sci Monit17201116 RA221-6
- G.L.HsuY.P.HungM.H.TsaiC.H.HsiehH.S.ChenE.Molodyskyet alPenile veins are the principal component in erectile rigidity: a study of penile venous stripping on defrosted human cadaversJ Androl33201211761185
- G.L.HsuH.S.ChenC.H.Hsiehet alInsufficient response to venous surgery: is penile vein recurrent or residual?J Androl272006700706
- V.MichalR.KramarJ.PospichalL.HejhalDirect arterial anastomosis on corpora cavernosa penis in the therapy of erective impotenceRozhl Chir521973587590
- R.ViragVasculogenic impotence. A review of 92 cases with 54 surgical operationsVas Surg151981917
- D.HauriA new operative technique in vasculogenic erectile impotenceWorld J Urol41986237243
- W.L.FurlowL.D.KnollR.C.BensonCurrent status of penile revascularization with deep dorsal vein arterialization: experience with 95 patientsInt J Impot Res2Suppl. 21990348
- C.RoweS.GanickMunarriz: traumatic vasculogenic erectile dysfunction. Role of penile microarterial bypass surgeryCurr Urol Report112010427431
- D.K.MontagueJ.H.BaradaA.M.BelkerL.A.LevineP.W.NadigC.G.Roehrbornet alClinical guidelines panel on erectile dysfunction: summary report on the treatment of organic erectile dysfunctionJ Urol156199620072011
- J.UdoErectile dysfunctionA.JardinG.W.KhouryS.GiulianoF.Padma-NathanR.Rosen1st International consultation on erectile dysfunction1999SpringerParis355404 Chapt. 10
- J.MulcahySexual medicine—sexual dysfunctions in men and womenT.F.LueR.B.RosenR.GiulianoF.KhouryF.Montorsi2nd International consultation on sexual dysfunctions2004Health Publications Ltd.Paris469 Chapt. 14
- D.K.MontagueJ.P.JarowG.A.BroderickR.R.DmochowskiJ.P.Heatonet alChapter 1. The management of erectile dysfunction: An AUA updateJ Urol1742005230239
- M.SohnA.Martin-MoralesSurgical treatment of erectile dysfunctionJ.B.PorstJ.BuvatStandard practice in sexual medicine2006WileyHoboken, NJ126148
- Y.KawanishiK.KimuraR.NakanishiK.KojimaA.NumataPenile revascularization surgery for arteriogenic erectile dysfunction: the long-term efficacy rate calculated by survival analysisBJU Int942004361368
- Y.VardiI.GruenwaldU.GedaliaS.NassarA.EngelY.Har-ShaiEvaluation of penile revascularization for erectile dysfunction: a 10-year follow-upInt J Impot Res162004181186
- O.KayigilK.AgrasE.OkuluIs deep dorsal vein arterialization effective in elderly patients?Int Urol Nephrol402008125131
- R.MunarrizJ.UberoiG.FantiniD.MartinezC.LeeMicrovascular arterial bypass surgery: long-term outcomes using validated instrumentsJ Urol1822009643648
- O.KayýgilE.OkuluM.AldemirE.OnenPenile revascularization in vasculogenic erectile dysfunction (ED): long-term follow-upBJU Int1092012109115
- A.R.BabaeiM.R.SafarinejadA.A.KolahiPenile revascularization for erectile dysfunction. A systematic review and meta-analysis of effectiveness and complicationsJ Urol6200917
- C.H.HsiehC.J.WangG.L.HsuS.C.ChenP.Y.LingT.Wanget alPenile veins play a pivotal role in erection: the hemodynamic evidenceInt J Androl2820058892
- F.ParonaImperfect penile erection due to varicosity of the dorsal vein: observationGiornale Italiano Delle Malattie Veneree E Della Pelle1418737176
- J.S.WootenLigation of the dorsal vein of the penis as a cure for atonic impotenceTexas Med J181902325328
- O.S.LowsleyA.RuedaFurther experience with an operation for the cure of certain types of impotenceJ Int Coll Surg1919536977
- E.WespesC.C.SchulmanVenous leakage. Surgical treatment of a curable cause of impotenceJ Urol1331985796798
- J.A.ValeM.R.FeneleyW.R.LeesR.S.KirbyVenous leak surgery. Long-term follow-up of patients undergoing excision and ligation of the deep dorsal vein of the penisBr J Urol761995192195
- M.CakanF.YalcinkayaF.DemirelT.OzgunayU.AltugIs dorsal penile vein ligation (DPVL) still a treatment option in veno-occlusive dysfunction?Int Urol Nephrol362004381387
- T.I.HwangC.R.YangPenile vein ligation for venogenic impotenceEur Urol2619944651
- G.L.HsuH.S.ChenC.H.Hsiehet alSalvaging penile venous stripping surgeryJ Androl312010250260
- R.C.NuR.Dean CarrionD.BochinskiT.F.LueCrural ligation for primary erectile dysfunction: a case seriesJ Urol173200520642066
- T.C.TsaiG.L.HsuS.C.ChenC.L.WangAnalysis of the results of reconstructive surgery for vasculogenic impotenceJ Formos Med Assoc871988182187
- C.H.HsiehS.P.LiuG.L.HsuH.S.ChenE.MolodyskyY.H.Chenet alAdvances in our understanding of mammalian penile evolution, human penile anatomy and human erection physiology: clinical implications for physicians and surgeonsMed Sci Monit182012RA11825
- G.L.HsuH.S.ChenC.H.HsiehW.Y.LeeK.L.ChenC.H.ChangClinical experience of a refined penile venous surgery procedure for patients with erectile dysfunction: is it a viable option?J Androl312010271280
- Hsu G.L. Physiological approach to penile venous stripping surgical procedure for patients with erectile dysfunction (Patent No.: US 8,240,313,B2). http://www.google.com/patents/US20110271966.
- V.F.S.TsaiH.C.ChangS.P.LiuY.C.KuoJ.H.ChenF.S.Jawet alDetermination of human penile electrical resistance and implication on safety for electrosurgery of penisJ Sex Med7201028912898
- S.C.ChenC.H.HsiehG.L.HsuC.J.WangH.S.WenP.Y.Linget alThe progression of the penile vein: could it be recurrent?J Androl2620055663
- G.L.HsuC.H.HsiehH.S.ChenP.Y.LingH.S.WenH.M.Huanget alThe advancement of pure local anesthesia for penile surgeries: can an outpatient basis be sustainable?J Androl282007200205
- S.ElhanblyS.Abdel-GaberH.FathyY.El-BayoumiM.WaldC.S.NiederbergerErectile dysfunction in smoker. A penile dynamic and vascular studyJ Androl252004991995