Abstract
Objective:
To evaluate serum levels of galectin-3 (G-3) in patients with bladder cancer and a control group, as a potential diagnostic and prognostic serum tumour marker.
Patients and methods:
Between November 2012 and January 2013, 55 patients (median age 58 years) were enrolled into three groups, i.e., a control, those with transitional cell carcinoma (TCC) or those with squamous cell carcinoma (SCC). The serum G-3 level was measured the night before cystoscopy. The levels of G-3 levels were correlated with tumour type, stage and grade, and in relation to levels in normal urothelium. The results were analysed statistically using the Mann–Whitney U-test, the Kruskal–Wallis test and the receiver operating characteristic curve, as appropriate.
Results:
The median serum G-3 level was 100, 720 and 920 pg/mL in the control, TCC and SCC groups, respectively, with very significantly greater G-3 levels in both the TCC and SCC groups than in the control group. Patients with high-grade TCC had a statistically significantly greater serum G-3 level than those with low-grade tumours, as did those with muscle-invasive TCC than those with Ta tumours.
Conclusions:
The level of G-3 can aid as a diagnostic marker in patients with either TCC or SCC of the bladder, but the prognostic significance of G-3 remains to be confirmed.
Introduction
Carcinoma of the bladder is still a worldwide health problem, and is ranked ninth in cancer incidence [Citation1]. Several urine and serum tumour markers have been studied for diagnosing this cancer, but a histopathological examination of transurethrally resected bladder tumour is the cornerstone of the diagnosis of bladder cancer, allowing both a histological diagnosis and tumour staging [Citation2]. The management of non-muscle-invasive (NMI) TCC of the bladder is based on frequent endoscopic examinations, together with urinary cytology, which has stood the test of time as a diagnostic and prognostic marker [Citation3].
The galectins are a group of proteins that regulate various biological cycles, including cell growth, cell differentiation, cell adhesion and apoptosis [Citation4]. Galectin-3 (G-3) shows pathological expression in many tumours, e.g., in human pancreas, colon and bladder cancer [Citation5,Citation6]. The intensity of expression depends on tumour progression, invasiveness and metastatic potential [Citation7]. High circulating levels of G-3 were also found to correlate with its anti-apoptotic activity as a possible cause for carcinogenesis [Citation8].
Thus we measured the levels of serum G-3 in patients with bladder cancer, both TCC and squamous cell carcinoma (SCC), and in control patients, to evaluate its role in the diagnosis of bladder cancer, and to correlate these levels with different tumour stages and grades.
Patients and methods
This prospective non-randomised study followed the tenets of the Declaration of Helsinki (1975) and had the approval of the ethics committee of the Theodor Bilharz Research Institute. The study initially included 75 patients (58 men and 17 women, median age 58 years, range 30–84) admitted to the urology department between November 2012 and January 2013. Fifty-four patients had a primary radiological diagnosis of bladder tumour for investigation, while 21 were enrolled as controls. After providing informed consent, a blood sample was taken from all patients the night before cystoscopy. The bladder tumour was resected transurethrally in all patients with apparent tumour, while cold-cup biopsies were taken from the control group, who were having a cystoscopy as part of another endourological procedure.
The samples were evaluated histopathologically by a uropathologist in the pathology department of the institute, according to the international histological classification of urinary bladder tumours proposed by the WHO in 2004.
About 2 mL of blood was collected under aseptic conditions the night before surgery, and the samples were delivered into a plain tube and allowed to clot. Serum was then removed, aliquoted into clean vials and stored frozen at −20 °C. An enzyme immunoassay technique was used to measure the serum level of G-3 (eBioscience, Vienna, Austria) [Citation9].
The results were expressed as the median (range). The variables were compared statistically between the two groups using the Mann–Whitney U-test, and among the three groups compared using the Kruskal–Wallis test followed by the Mann–Whitney U-test. A receiver operating characteristic (ROC) curve was used to determine the sensitivity and specificity of serum G-3 for detecting cancer. In all tests P < 0.05 was considered to indicate significance and P < 0.01 was considered highly significant.
Results
From the 54 patients with a radiologically diagnosed bladder growth, seven had normal findings on cystoscopy and two had bilharzial granulomas, so all nine were excluded. The histopathological evaluation of the control arm showed cystitis in 11 of the 21 patients and they were also excluded. The final results were obtained from 55 patients, divided into three groups, i.e., those with normal urothelium, those with SCC and those with TCC. The clinicopathological distribution is shown in .
Table 1 The clinicopathological distribution in the three groups.
For an accurate evaluation of the serum G-3 level, patients with NMI TCC were classified according to the depth of invasion into those with Ta tumours (six) and those with T1 tumours (11).
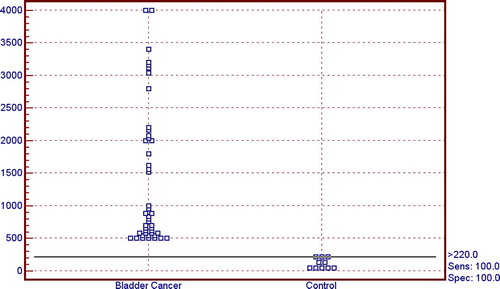
The serum G-3 levels in the various groups are shown in . The level was significantly higher in both the TCC and SCC groups than in the controls, with no significant difference between the TCC and SCC groups (P > 0.05; ).
Table 2 G-3 levels in the study groups.
The serum G-3 level was significantly higher in MI tumours than in papillary noninvasive tumours (Ta; P < 0.05). There was no significant difference between MI tumours and tumours invading the subepithelial connective tissue (T1), nor between papillary noninvasive tumours (Ta) and T1 tumours (P > 0.05; ). There was a significantly higher G-3 level in high-grade than in low-grade tumours (P < 0.05).
A ROC curve () was used to determine the threshold serum G-3 level for diagnosing bladder cancer in relation to the normal control. The threshold level of serum G-3 at ⩾220 pg/mL gave the best specificity (100%) for diagnosing bladder cancer (either TCC or SCC) with 100% sensitivity, and positive and negative predictive values and diagnostic accuracy of 100%.
Discussion
Several tumour markers were studied in a trial to improve the detection rate of bladder cancer, using non-invasive techniques [Citation3], but none have yet taken the place of urinary cytology, which showed up to 85% sensitivity and 87% specificity for high-grade tumours [Citation10].
G-3, an intracellular secreted carbohydrate-binding protein, acts as a regulator of the cell cycle, inflammation, immune responses, cell adhesion, and cell signalling events [Citation11,Citation12]. Anti-apoptotic activity was attributed as a possible cause for the involvement of G-3 in carcinogenesis [Citation13].
In the present study the serum G-3 level was significantly higher in patients with bladder cancer, both TCC and SCC (all stage-confined) than in the controls. Similar results were found by Waalkes et al. [Citation4], who showed that patients with a bladder tumour had a high serum level of G-3, suggesting the potential role of G-3 levels in the diagnosis of bladder cancer. There was no statistically significant difference in the G-3 serum level between TCC and SCC.
In 2008, Sakaki et al. [Citation14] reported no significant statistical difference in serum G-3 levels in patients with NMI and MI tumours. In the present study the serum G-3 level was statistically significantly higher in patients with MI TCC than in those with Ta tumours, but this significance was lost when comparing MI tumours to tumours invading the lamina propria (T1), and when comparing T1 to Ta. This difference might be because in the present study we measured G-3 levels separately in patients with Ta or T1 tumours.
There were statistically significantly greater serum G-3 levels in high-grade than in low-grade TCC of the bladder; this must be evaluated further to determine its validity and prognostic significance.
The detection rate for bladder cancer was 100% using a threshold G-3 value of 220 pg/mL, but these results must be taken cautiously because of the small sample, and that there were only 10 patients in the control arm. The effect of urothelial inflammation on the G-3 level in relation bladder cancer must also be considered.
This study had several limitations, as although it was prospective, it was not randomised, and it included patients with a first diagnosis of bladder cancer, so we did not assess the pattern of G-3 levels in patients who had received intravesical treatment for NMI TCC.
In conclusion, the serum G-3 level is sensitive and specific for the diagnosis of both TCC and SCC of the bladder, but this prognostic significance should be confirmed. Large-scale randomised studies are needed to confirm these results and to evaluate G3 levels in patients treated for NMI bladder cancer.
Conflict of interest
None.
Source of funding
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- R.SiegelD.NaishadhamA.JemalCancer statistics, 2013CA Cancer J Clin6320131130
- Mitra AP. Urine cytologic analysis: special techniques for bladder cancer detection. In: Kumar GL, Kiernan JA, editors. Connection, Carpinteria, CA: Dako; 2010 (14), p. 169–177.
- Y.FradetH.GrossmanL.GomellaS.LernerM.CooksonD.Albalaet alA comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter studyJ Urol17820076873
- S.WaalkesA.MerseburgerA.SimonJ.SerthM.KuczykGalectin expression in urological cancer. Diagnostic, prognostic and therapeutic potentialUrologe A492010387391
- Y.TakenakaH.InoharaT.YoshiiK.OshimaS.NakaharaS.Akahaniet alMalignant transformation of thyroid follicular cells by galectin-3Cancer Lett1952003111119
- A.KrzeslakA.LipinskaGalectin-3 as a multifunctional proteinCell Mol Biol Lett92004305328
- P.Nangia-MakkerV.BalanA.RazRegulation of tumor progression by extracellular galectin-3Cancer Microenviron120084351
- V.BalanP.Nangia-MakkerA.RazGalectins as cancer biomarkersCancers22010592610
- H.SanoD.K.HsuL.YuJ.R.ApgarI.KuwabaraT.Yamanakaet alHuman galectin-3 is a novel chemoattractant for monocytes and macrophagesJ Immunol165200021562164
- P.MoonenG.MerkxP.PeelenH.KarthausD.SmeetsJ.A.Witjeset alUroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle invasive bladder cancerEur Urol51200712751280
- M.ElolaW.TodelM.TroncosoG.VastaG.RabinovichGalectins. Matricellular glycan-binding proteins linking cell adhesion, migration, and survivalCell Mol Life Sci64200716791700
- G.VastaRoles of galectins in infectionNat Rev Microbiol72009424438
- Y.PierreGalectins in hematological malignanciesAm J Blood Res12011119129
- M.SakakiN.OkaR.NakanishiK.YamaguchiT.FukumoriH.O.Kanayamaet alSerum level of galectin-3 in human bladder cancerJ Med Invest552008127132