Abstract
Objectives:
To report the findings and management of patients with persistent Müllerian duct syndrome (PMDS).
Patients and methods:
Nineteen phenotypically male patients (aged 8 months to 27 years) presented with testicular maldescent. All of them had normal male external genitalia. Two of them had had a previous diagnosis of persistent Müllerian structures. All patients were karyotyped, and had a hormonal profile, diagnostic laparoscopy, retrograde urethrocystogram, gonadal biopsies, and surgical management according to the findings. The follow-up was based on a clinical examination, abdominal ultrasonography (US) and scrotal colour-Doppler US at 3 and 6 months after surgery, and every 6 months thereafter.
Results:
Diagnostic laparoscopy showed the presence of persistent Müllerian structures in all 19 patients. All patients had a normal male karyotype (46XY). Ten patients had a laparoscopic excision of their Müllerian structures while the remaining nine patients had their Müllerian structures left in place. No malignant changes were found in the excised Müllerian tissues. Of the 37 gonadal biopsies taken, 31 (84%) indicated normal testes.
Conclusions
The incidence and prevalence of PMDS are not well estimated. Müllerian structures should be removed whenever possible to avoid the risk of malignant transformation. The early diagnosis of PMDS makes possible the excision of Müllerian structures and a primary orchidopexy. A long-term follow-up is needed for patients with intact Müllerian structures and magnetic resonance imaging might be a better method than US for that purpose. Most of the patients had normal testicular histology, which might allow fertility.
Introduction
The persistent Müllerian duct syndrome (PMDS), classically known as hernia uteri inguinalis, is a rarely reported disorder of sexual development (DSD), first described by Nilson in 1939 [Citation1]. In this condition, genetically and phenotypically male subjects develop female internal organs (uterus and Fallopian tubes) due to a deficiency in the anti-Müllerian hormone (AMH) produced by Sertoli cells, or its type II receptor (AMHR-II), and it has an autosomal recessive mode of inheritance. Clarenette et al. [Citation2] suggested that there were three subgroups, i.e., 60–70% of cases have intra-abdominal Müllerian structures and testes in a position simulating that of the ovaries, 20–30% have one testis in a hernial sac or scrotum together with Müllerian structures, and 10% have both testes located in the same hernial sac (transverse testicular ectopia) along with the Fallopian tubes and uterus. Ultrasonography (US), MRI and multi-detector CT successfully detected the persistent Müllerian structures with no clear advantage of one over the others, as there are too few cases and a lack of comparative studies [Citation3,Citation4]. Laparoscopy is by far the most accurate diagnostic method for the impalpable testis (up to 97% accuracy) [Citation5]. The association of cryptorchidism and PMDS makes laparoscopy the method of choice for both the diagnosis and treatment. A considerable percentage of all reported cases was found to have malignant transformation of their Müllerian structures [Citation6–Citation16]. Hence, all such structures should be removed whenever possible to eliminate the risk of malignancy and to save the patient a lifelong follow-up. In the present study we report the clinical findings and management of 19 patients with PMDS.
Patients and methods
From June 2009 to August 2013, 19 phenotypically male patients (mean age 8.2 years, range 8 months to 27 years) presented with testicular maldescent. All of them had normal male external genitalia. Two patients had had a previous right inguinal orchidopexy; one of them was assessed using MRI (to visualise his left impalpable testis), and this detected a uterus. Another patient had surgery for a left inguinal hernia, during which a lump was excised and confirmed to be uterine tissue. All patients were karyotyped and had a hormonal profile (FSH, luteinising hormone, LH, and testosterone), a diagnostic laparoscopy which confirmed the presence of Müllerian structures, a retrograde urethrocystogram, gonadal biopsies, and surgical management according to the findings. Three adult patients had semen analysed and tumour markers assessed before surgery, i.e., human chorionic gonadotrophin (hCG), α-fetoprotein (AFP), and lactate dehydrogenase (LDH).
To excise the Müllerian structures laparoscopically, three ports were used, i.e., an 11-mm camera port at the umbilicus, a 12-mm right-hand port in the right iliac fossa midway between the umbilicus and the anterior superior iliac spine, and a 6-mm left-hand port in a mirror position to that of the right hand. The procedure started by clipping the left Fallopian tube and gubernaculum a few centimetres below the lower pole of the left testis, then the vessels traversing the left broad ligament were secured. The left vas was then dissected gently away from the uterus down to a point where the two vasa seemed to be tightly adherent to the lowermost part of the cervix uteri. The cervix was then isolated and clipped above that point and cut. The uterus was dissected away from the right vas deferens, which was closer and more adherent to the structures than the left vas in all 10 patients. The vessels in the right broad ligament were then secured and the right Fallopian tube and gubernaculum were clipped and cut. Excised tissues were extracted directly through the right-hand port. A Prader orchidometer was used to measure testicular volume at the time of the orchidopexy. The follow-up was based on a clinical examination, abdominal US and scrotal colour-Doppler US at 3 and 6 months after surgery, and every 6 months thereafter.
An independent sample t-test was used to compare the mean ages of the patients who had their Müllerian structures excised and those who had them left intact. A paired sample t-test was used to compare the means of testicular volumes, whilst the chi-squared test was used to assess the correlation between the method of orchidopexy and the management of the Müllerian structures.
Results
Before surgery, the clinical examination showed that 14 patients (74%) had bilateral impalpable testes whilst four (21%) had a left impalpable testis; two of them had had a previous right inguinal orchidopexy, one a right inguinal testis and one a right scrotal testis. One patient (5%) had a right impalpable and left inguinal testis. All 19 patients had a normal 46 XY karyotype, 17 of them (90%) had normal hormone levels, whilst two adult patients had abnormal hormonal profiles (one had high FSH and LH levels and the other had high FSH levels). All three adult patients had azoospermia; two of them had spermatogenic cells in semen (100,000/mL and 200,000/mL). These three had normal tumour marker levels (hCG < 5 mIU/mL, LDH < 200 U/L, AFP < 10 ng/mL).
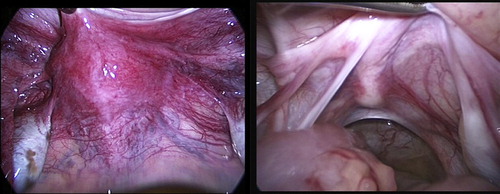
On diagnostic laparoscopy, all 19 patients had persistent Müllerian structures (uterus and Fallopian tubes). The most common initial finding was an abnormally raised peritoneal fold tenting over the bladder, and the gubernacula seemed to be joining in the midline and the testes were medial to the external iliac vessels. Of the 14 patients with bilateral impalpable testes, 13 had bilateral abdominal testes and one had a left abdominal testis and right atrophic gonad. Of the four patients with a left impalpable testis, two had a left abdominal testis, one a left atrophic gonad and the fourth who had a right scrotal testis was found to have left transverse testicular ectopia (the left testis in the right scrotal compartment) and a ‘vanishing’ right testis. The patient with a right impalpable and left inguinal testis had a right abdominal testis. All testes found appeared to be almost normal except for some small cystic areas on the surface filled with yellowish-brown fluid (). Twenty-seven (90%) of the 30 abdominal testes found were medial and below the level of the external iliac vessels, whilst the remaining three (10%) were high abdominal. The retrograde urethrocystograms were normal in all 19 patients.
Operative results
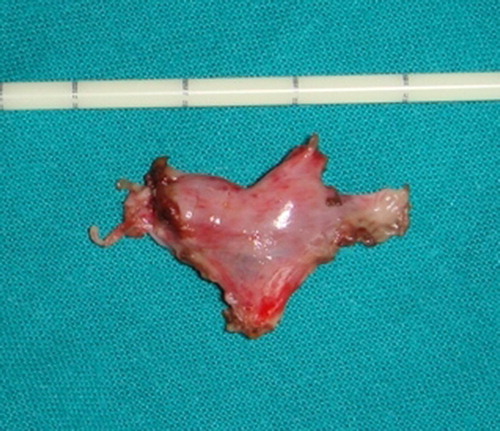
Ten patients had a laparoscopic excision of their Müllerian structures (), seven of them had a bilateral laparoscopic primary orchidopexy, two of them had a left laparoscopic primary orchidopexy (both had a previous right inguinal orchidopexy elsewhere) and one had excision of a left atrophic gonad and a right laparoscopic primary orchidopexy for his inguinal right testis. The remaining nine patients had their Müllerian structures left in place, six of them had a bilateral laparoscopic staged Fowler-Stephens orchidopexy (FSO), one had a left primary and a right staged FSO, one had excision of a right atrophic gonad and a left staged FSO and the patient with the left transverse testicular ectopia had only a gonadal biopsy (). During 32 orchidopexies there were two (6%) intraoperative complications, both being an inadvertently cut left vas deferens during primary orchidopexy, one of them due to a difficult dissection because of a previous left inguinal hernia repair. From the Prader orchidometer the mean (range) testicular volume for the 35 testes was 3.5 (1–15) mL. Gonadal biopsies were obtained during diagnostic laparoscopy for the first seven cases and then during the orchidopexy afterwards. Excisional biopsies were designed to include the grossly abnormal cystic areas in all gonads but the three scrotal testes were assessed using fine-needle aspiration cytology. The two atrophic gonads were excised and assessed for histopathology.
Table 1 A comparison of the age of patients who had their Müllerian structures excised with the age of those who had them left intact.
Histopathology
Of the 37 biopsies taken, 31 (84%) showed normal testes, four (11%) had Sertoli-cell-only syndrome (two patients) and the two atrophic gonads (5%) were streak gonads. Histopathological examination of the 10 excised Müllerian structures showed infantile uterine tissue and Fallopian tubes.
Follow-up
The mean (range) follow-up was 18.2 (3–42) months and clinically, all 32 operated testes retained their scrotal positions. One right testis was high scrotal; the patient was a 5-year-old who had the Müllerian structures excised and a bilateral primary orchidopexy. From the Prader orchidometer the range of volume remained 1–15 mL for the 35 testes but the mean volume increased to 4.3 mL. Abdominal US showed no abnormalities in all patients during the follow-up. Müllerian structures were visible in four patients of the nine who retained them. Scrotal colour-Doppler US at 3 months showed normal echogenicity and blood flow for the 35 testes, and a mean (range) volume of 3.1 (0.43–14.6) mL, and at the latest follow-up this was 3.9 (0.54–14.7) mL (). All 16 prepubertal patients had testicular volumes conforming to the 10th or the 50th centiles of their ages on the reference curves constructed by Mul et al. [Citation17], whilst the testicular volumes for the three adults were below the 10th centiles. Tumour marker levels for the three adult patients remained normal during the follow-up.
Table 2 A comparison of the initial to final testicular volumes (measured by the Prader orchidometer) and scrotal US.
Discussion
We diagnosed 19 cases of PMDS of 832 patients with impalpable testes in whom we used diagnostic laparoscopy during the same period of the study (2.3%). Since the first report of PMDS, ≈262 cases have been reported. Many cases might have been missed in the era before laparoscopy, in which almost all cases reported were of the ‘hernia uteri inguinalis’ variant, which represent 20–30% [Citation2]. This might indicate that the incidence and prevalence of this condition are not well estimated. The results of the present study showed that prepubertal patients with PMDS had testicular volumes conforming to the reference curves for normal testes [Citation17], and their testes showed better compensatory growth than those of adult patients after orchidopexy, which might support the theory that testicular damage is due to neglected cryptorchidism rather than a part of the syndrome. We noticed that the vas deferens was abnormally tortuous in all cases, but the presence of spermatogenic cells in the semen of two patients excluded obstruction. Another concern is the malignant transformation of the Müllerian tissues. Of the 262 reported cases, 11 had malignancies of their Müllerian structures [Citation6–Citation16]. Farikullah et al. [Citation3] constructed a statistical model from these reports, assuming that malignancy was binomially distributed, and concluded that 3.1–8.4% of males with PMDS will develop Müllerian malignancies. Such reports might not be a representative sample because of the heterogeneity of the disorders reported, but they support the argument that Müllerian structures should be excised whenever possible. In the present study all the excised Müllerian tissues were free of malignancy and those left intact were not suspect, but we noticed that they were larger and more vascular in older patients (). This type of development (rather than involution) warns against possible complications and justifies excision. More recent reports suggested that the persistence of Müllerian structures did not add to the risk of testicular cancer attributed to the associated cryptorchidism [Citation3]. The risk of testicular malignancy increases by 35–50 times with cryptorchidism [Citation18]. If such values are multiplied by the highest incidence of testicular cancer according to Cancer Research UK (10.7 per 100,000 in Denmark), the estimated incidence of testicular cancer among cryptorchid patients will be 535 per 100,000. If compared to the estimated incidence of Müllerian malignancy among patients with PMDS (3.1–8.4%), this means that a patient with PMDS is at a greater risk of Müllerian malignancy than that of testicular cancer [Citation3,Citation19] Chan et al. [Citation20] recently reported that orchidopexy before the age of 10–11 years might eliminate the risk of testicular malignancy associated with cryptorchidism. Such data, together with our findings of statistically significant age difference between patients with excised structures and those who kept them, highlight the value of an early intervention in PMDS. The aim of our management was to preserve the testes, and hence the fertility potential, to excise the abnormal Müllerian structures whenever possible, reassure the parents (and/or the patients) about gender identity, and explain the difference from other DSD, especially true hermaphroditism which is a social myth in our community. The first idea was that cryptorchidism was due to misdirection and anatomical obstruction by the abnormal connection to the Müllerian structures, and not an actual shortening of the internal spermatic vessels or vas deferens [Citation18], and so excision of the structures and primary laparoscopic orchidopexy should have been possible in all cases. In the present study most of the patients presented with neglected cryptorchidism (mean age 8.2 years) and short internal spermatic vessels were found, which necessitated a staged FSO. It was not possible to preserve the gubernaculum during excision of the Müllerian structures, and dissection of the vas deferens away from them would have risked the vasal vessels, the remaining pedicle for the testis in such cases. After counselling the parents (and/or the patients) about the greater possibility of testicular atrophy if the FSO and excision of the Müllerian structures were done together, and the need for a long-term follow-up if the structures were left intact, our decision was to leave the Müllerian structures in place whenever FSO was necessary. To our knowledge, this is the largest report of patients with ‘pure’ PMDS, especially after excluding two patients with hypospadias (which might refer to gonadal dysgenesis) and one true hermaphrodite. We understand that the limitations of this study were the lack of AMH levels for our patients and that we did not do any genetic testing for the AMHR-II gene, and this was mainly for financial reasons. A longer follow-up is needed, especially for those patients who kept their Müllerian structures intact. We recommend that more efforts should be directed towards spreading an awareness of DSD associated with cryptorchidism among primary-care physicians and patients. All patients with an impalpable testis should undergo diagnostic laparoscopy, especially before the management of the other inguinal testis. Surgeons should look for the signs of PMDS during a diagnostic laparoscopy, i.e., the tenting peritoneal fold on the back of the bladder, testes medial to the external iliac vessels, and gubernacula meeting in the midline.
Figure 3 The size and vascularity of Müllerian structures from 15-month-old (right) and 22-year-old (left) patients.
In conclusion, the incidence and prevalence of PMDS are not well estimated. Müllerian structures should be removed whenever possible to avoid the risk of malignant transformation and the need for a longer follow-up. The need for a FSO prevents excision of the Müllerian structures. The early diagnosis of PMDS makes excision of Müllerian structures and primary orchidopexy possible. A long-term follow-up is needed for patients with intact Müllerian structures, and MRI might be a better imaging method than US for that purpose. Most of the patients have normal testicular histology, which might allow fertility.
Conflict of interest
None.
Source of funding
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- O.NilsonHernia uteri inguinalis beim ManneActa Chir Scand831939231
- T.D.ClarnetteY.SugitaJ.M.HutsonGenital anomalies in human and animal models reveal the mechanisms and hormones governing testicular descentBr J Urol79199799112
- J.FarikullahS.EhtishamS.NappoL.PatelS.HennayakePersistent Müllerian duct syndrome: lessons learned from managing a series of eight patients over a 10-year period and review of literature regarding malignant risk from the Müllerian remnantsBJU Int1102012E1084E1089
- A.ClementeV.MacchiM.BerrettaA.MorraA female form of persistent Müllerian duct syndrome: MDCT findingsClin Imaging322008314317
- R.G.MooreC.A.PetersS.B.BauerJ.MandellA.B.RetikLaparoscopic evaluation of the nonpalpable tests: a prospective assessment of accuracyJ Urol1511994728731
- J.P.XingJ.G.DangD.P.WuQ.Z.LongX.F.ChenX.Y.NanPapillary cystadenocarcinoma in a Müllerian duct cyst: report of a case with literature reviewZhonghua Nan Ke Xue122006218221
- S.W.WarmannM.VogelM.WehrmannH.G.Scheel-WalterA.ArtlichP.L.Pereiraet alGiant Müllerian duct cyst with malignant transformation in 15-year-old boyUrology672006424e3424e6
- D.D.ThielM.J.ErhardUterine adenosarcoma in a boy with persistent Müllerian duct syndrome: first reported caseJ Pediatr Surg402005e29e31
- F.R.RomeroM.FucsM.G.CastroC.R.GarciaC.Fernandes RdeM.D.PerezAdenocarcinoma of persistent mullerian duct remnants: case report and differential diagnosisUrology662005194195
- Y.ShinmuraT.YokoiY.TsutsuiA case of clear cell adenocarcinoma of the Müllerian duct in persistent mullerian duct syndrome: the first reported caseAm J Surg Pathol26200212311234
- S.KatoH.ItoK.KobayashiSquamous cell carcinoma in a Müllerian duct cyst: report of a caseSurg Today261996645648
- R.F.GilbertJ.IbarraL.A.TanseyA.M.ShanbergAdenocarcinoma in a Müllerian duct cystJ Urol148199212621264
- G.G.YoungsonSquamous metaplasia complicating a Müllerian duct remnantBr J Urol651990211212
- R.W.NovakR.B.RainesA.N.SolleeClear cell carcinoma in a Müllerian duct cystAm J Clin Pathol761981339341
- N.B.HodgsonLong-term survival from Müllerian duct carcinomaJ Urol1161976313315
- G.C.SzemesD.J.RubinSquamous cell carcinoma in a Müllerian duct cystJ Urol10019684043
- D.MulA.M.FredriksS.van BuurenW.OostdijkS.P.Verloove-VanhorickJ.M.WitPubertal development in The Netherlands 1965–97Pediatr Res502001479486
- J.M.HutsonS.HasthorpeC.F.HeynsAnatomical and functional aspects of testicular descent and cryptorchidismEndocr Rev181997259280
- CRC UK. Testicular cancer incidence statistics 2010 Available at: http://www.cancerresearchuk.org/cancer–info/cancerstats/types/testis/incidence/cited 30–3, 2014.
- E.ChanC.WayneA.NasrFRCSC for Canadian Association of Pediatric Surgeon Evidence-Based Resource. Ideal timing of orchiopexy: a systematic reviewPediatr Surg Int3020148797