Abstract
Objective:
To review the mode of presentation and clinical course of patients with prostate cancer during a specified period, as the detection rate is tending to increase, with most patients presenting at an advanced stage, and yet the overall incidence and prevalence rates are low.
Patients and methods:
We retrospectively reviewed all aspects of care for patients who were diagnosed between May 2006 and July 2010.
Results:
In all, 76 men had a histologically confirmed prostatic adenocarcinoma diagnosed between May 2006 and July 2010 (mean age 71.1 years, SD 8). The median (range) prostate-specific antigen level at diagnosis was 52 (1.2–16,230) ng/mL. Of the patients, 74% had a Gleason grade of ⩾ 7 on diagnosis, and 64% had extraprostatic disease on presentation. Active surveillance was adopted in four patients, and four others were maintained on watchful waiting. Six patients had a radical prostatectomy, in one of whom it was a salvage procedure. Six patients received external-beam radical radiotherapy, five of whom had neoadjuvant, concurrent and adjuvant hormonal therapy. All remaining patients were treated primarily with androgen-deprivation therapy (ADT). Of the patients on hormonal manipulation, in 56% the cancer became castrate-resistant within the mean (SD) follow-up of 17.2 (15) months. Of patients treated primarily with ADT, 34% died. The death rate among the whole group was 23%. Both percentages include both prostate cancer-specific and non-specific mortality.
Conclusion:
An advanced stage of disease at presentation mandates an early-detection, hospital-based screening programme. Further research should include many more patients and be based in several centres.
Introduction
It appears from the available data that prostate cancer has a low crude incidence rate that varies according to the geographical location, and is 2.6–3.5 per 100,000 with an increasing trend in the detection rate [Citation1–Citation3]. The formal use of PSA testing as a screening or early-detection tool is not currently practised in Saudi Arabia. The present authors’ tertiary-care centre is one of the major hospitals in the Eastern province of Saudi Arabia, which has a total population of around four million. It is apparent that a considerable number of patients presented with disease at an advanced stage. Based on this, the main objective of the present study was to review the mode of presentation and the clinical course of patients with prostate cancer referred to or diagnosed at our hospital in a specified period, with a view to improving the outcome.
Patients and methods
We retrospectively reviewed all aspects of the care of patients who were diagnosed with prostate cancer between May 2006 and July 2010, including the diagnosis, staging and treatment. Of these patients, 65 were referred from other hospitals after having a high PSA level or a biopsy diagnosis, while the remaining 11 were identified through the PSA screening of men aged >50 years who were assessed in our urology clinic, with or without LUTS. All records of the identified patients were reviewed. The standard biopsy protocol comprised 12-core biopsies taken from the peripheral zone (apical, intermediate and basal) under local anaesthetic. In patients referred after a biopsy taken in a secondary-care hospital, the number of biopsy cores was 6–12.
Staging was based on a DRE and imaging (MRI, CT and bone scan), all in correlation. The TNM (2002) staging system was the basis for staging. Organ-confined disease was defined as tumour that was localised within the prostatic capsule with no lymph node or distant metastasis. The mean (SD) follow-up was 17.5 (15) months.
Results
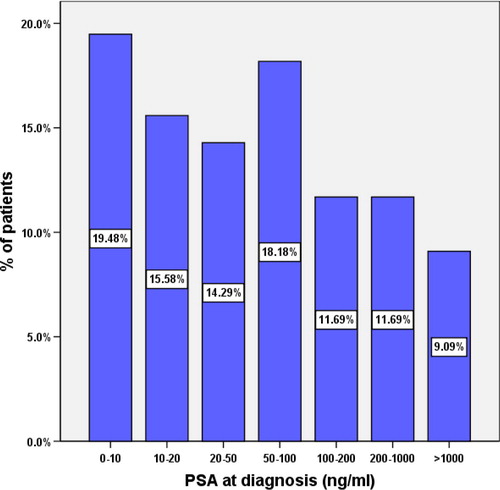
In all, 76 patients, with a mean (SD, range) age of 71.1 (8, 49–89) years, were diagnosed histologically to have prostatic adenocarcinoma during the specified period. Of these, 73 patients were diagnosed after a TRUS-guided biopsy indicated by a high PSA level or a suspicious DRE, or both, while three were diagnosed after TURP. The median (range) PSA level at diagnosis was 52 (1.2–16,230) ng/mL, with 65% of the patients having a PSA level of >20 ng/mL on presentation and 9% of >1000 ng/mL ().
Based on the Gleason grade, 74% of the patients were at least at moderate risk, with a Gleason score of 7 (). Of the 76 patients in the study, 72 had fully validated clinical staging data, qualifying them to be included in a further analysis which subsequently showed that 64% had extraprostatic disease on presentation. The metastatic pattern on presentation showed that 52% of patients who had a bone scan (total 63) were positive for bone metastases. In all, 54 patients had either staging CT or MRI, of whom half had lymph-node metastasis, 22% had pulmonary metastasis, and 11% had liver metastasis.
Table 1 The Gleason grades of the patients.
For treatments, active surveillance was adopted in four patients with low-risk disease that fulfilled the agreed criteria, and considering the patients’ preferences. All of these patients had a Gleason score of 6 with a PSA level of <10 ng/mL and a maximum clinical stage of T2a. In their follow-up regimen these patients had their PSA level measured and a DRE every 6 months, and a repeat prostatic biopsy every year. The PSA value and kinetics (velocity and doubling time) were also considered and a threshold value of PSA of 10 ng/mL was considered to indicate progression. They all remained stable with no disease progression during the specified follow-up period. Watchful waiting was adopted in four other patients who had significant comorbidities but no metastasis on presentation. They were maintained on watchful waiting to defer the undesirable side-effects of androgen-deprivation treatment (ADT), as they had mild symptoms or were totally asymptomatic but with associated comorbidities. The absence of symptoms was taken as a guide to continuing watchful-waiting, considering the PSA status. Six patients had a radical prostatectomy, in one of whom it was a salvage procedure after external-beam radiotherapy. Of these six patients, three had a good oncological outcome with an undetectable PSA level (<0.2 ng/mL), two were lost to follow-up, and one developed a biochemical relapse. The patient who had a salvage prostatectomy was 55 years old. His disease was initially staged as T3, with a PSA level of 23 ng/mL and Gleason score of 8. He was treated with radical radiotherapy, along with neoadjuvant, concomitant and adjuvant hormonal therapy. His nadir PSA level of 1.9 ng/mL was reached after 7 months. After that, his PSA level started to increase until it reached 26 ng/mL even with complete androgen blockage. He was offered a salvage prostatectomy but he remained hesitant for 17 months. His immediate preoperative staging showed organ-confined disease, but he developed a bone metastasis at 8 months after surgery and continued on ADT. Six patients received external-beam radiotherapy with curative intent, five of whom had neoadjuvant, concurrent and adjuvant hormonal therapy. One patient was declared as ‘do not resuscitate’ shortly after diagnosis, so no active form of treatment was initiated. All other patients were treated primarily with ADT. Our ADT protocol starts with the oral anti-androgen flutamide for 3 weeks, simultaneously with the first injection of an LH–RH analogue, either subcutaneous goserelin or intramuscular leuprolide, depending on patient’s preference after counselling. All patients then receive a 3-monthly LH–RH analogue on a continuous basis. Complete androgen blockade is implemented once PSA progression is detected. The next step was to withdraw the anti-androgen on further progression, and in 56% of patients primarily treated with hormonal manipulation the disease became castrate-resistant within the follow-up of 17.2 (15) months, and they were referred to oncologists for the consideration of chemotherapy. Of these patients primarily treated by hormonal therapy, 34% died, with an overall mortality rate of 23%, including both prostate cancer-specific and non-cancer-specific mortality.
Discussion
Since the introduction of PSA assays into clinical practice the mode of clinical presentation of prostate cancer has dramatically changed towards earlier disease stages [Citation4–Citation7]. This phenomenon of downward stage migration has become more evident in countries with widespread PSA testing, either as a population-based screening programme or as opportunistic early detection. Compared to previous studies documenting the metastasis rate on presentation [Citation8–Citation12] our values are the highest. Being a tertiary-care centre it was very difficult for us to track the interval between the onset of symptoms and the first contact with primary healthcare, and whether the PSA testing was requested by the patient, either symptomatic or asymptomatic, or by the primary healthcare personnel. In this regard a previous study exploring the knowledge and attitude of men from Saudi Arabia, and neighbouring Egypt and Jordan, showed that participants in the three countries shared the common characteristics of poor knowledge and a fair attitude towards prostate cancer examination and screening practice [Citation13].
The PSA testing in the present study was perceived as a means of early detection with no intention to address the effect on mortality, similar to the landmark studies of the Prostate, Lung, Colorectal and Ovarian (PLCO) [Citation14] and European Randomized Study of Screening for Prostate Cancer (ERSPC) [Citation15], with all the controversies around them [Citation16]. Nevertheless, one of the subsidiary studies from the ERSPC showed that PSA screening significantly decreased the risk of developing metastasis [Citation7].
Our hospital is a tertiary-care centre, considered a referral hospital for patients with cancer, and is one of the largest hospitals in the Eastern province of Saudi Arabia, which has a population of around four million. However, the relatively few patients with prostate cancer in the present study (compared to Western values) reflect the true low incidence and prevalence of the disease. Earlier studies from the local area (Eastern province in Saudi Arabia) in 1987 (before the use of PSA) did not include prostate cancer in the top five male cancers [Citation17]. Later, in 1997, Mosli [Citation8] reviewed prostate cancer in Saudi Arabia in terms of epidemiology and outcome. In his conclusion, he stated that the prevalence of prostate cancer was low, but anticipated that the detection rate would increase because of the ageing population, and increased rate of TURP and of PSA testing. Statistics from the Saudi Prostate Cancer Management Guidelines group [Citation2] showed that a total of 1869 cases of prostate cancer were recorded in Saudi Arabia between 1994 and 2004. The number of cases has been increasing steadily, from 151 during 1994 to a peak of 243 during 2004. A recent study by Alghamidi et al. in 2013 [Citation1] showed that the incidence is higher than expected, with an age-standardised incidence rate of up to 10.1 in the eastern region (the same geographical location of the present study). One further study by Al-Abdin et al. [Citation18] investigated the detection rate of prostate cancer in a cohort of Saudi men compared to their Canadian counterparts. It was evident from their report that there was a significantly low detection rate of prostate cancer in the Saudi group even with PSA values as high as 10 ng/mL, which suggested an increase in the threshold for taking a TRUS-guided biopsy. One epidemiological study of prostate cancer from United Arab Emirates [Citation19] also showed a low prevalence. In that study, 40% of patients were originally from neighbouring Arab countries such as Syria, Yemen, Oman and Jordan. One further study assessed the cancer epidemiology and control in the Arab world [Citation20], showing that the most prevalent male cancers were of the lung, urinary bladder and liver. In the same study the following was quoted ‘Adenocarcinomas of the breast, prostate and colorectum appear to be increasing’. With regard to tumour characteristics, such as PSA values, Gleason grading and metastatic pattern, the present study showed that a small percentage could be described as clinically insignificant cancers. One previous study from Saudi Arabia on the clinicopathological patterns of prostate disease and prostatic cancer concurred with our findings [Citation21]. It showed that more than half of the patients (53%) presented with a PSA level of >20 ng/mL, and 63% to have a Gleason grade of 7. Another study from the region reported difficulty in applying the Epstein criteria for clinically insignificant prostate cancer in patients in the Middle East, as the final pathology results after radical prostatectomy were more unfavourable than they were thought to be in 46% who initially fulfilled the criteria [Citation22]. Furthermore, the metastatic pattern in the present patients was peculiar in terms of soft-tissue involvement, as 50% had lymph-node metastasis, 22% had pulmonary metastasis, while 11% had liver metastasis on presentation. Soft-tissue metastasis on its own constitutes a poor prognostic criterion [Citation23].
There is a need to adopt a strategy of trying to diagnose prostate cancer in earlier curable stages. Adopting a population-based screening programme will not be cost-effective in Saudi Arabia, because of the apparent low prevalence of the disease. Even in the USA, where the disease has a high prevalence, the PLCO screening study concluded that prostatic carcinoma-related mortality rates were very low and with no significant difference between a screening group and a standard-care group [Citation14]. It might be a more reasonable approach to adopt a hospital-based early-detection programme. This can be done in urology as well as non-urology clinics. This programme will be valid for the symptomatic group of patients who present with LUTS that could be related to BPH or chronic prostatic inflammation, both of which have an association with prostatic cancer [Citation24–Citation27]. In this group of patients, a DRE and PSA testing is definitely recommended.
In the group of patients with no LUTS or BPH it would be prudent to adopt the same guidelines of the European Association of Urology or the AUA for the early detection of prostatic cancer [Citation28,Citation29]. The European Association of Urology guidelines recommend a baseline PSA determination at age 40 years, upon which the subsequent screening interval might then be based [Citation29]. The optimal schedule of this screening has not been yet standardised. The AUA guidelines indicated that a routine screening interval of 2 years might be preferred over annual screening in those men who have participated in shared decision-making and decided to opt for screening. The Panel recommended shared decision-making for men aged 55–69 years and considering PSA-based screening, a target age group for whom the benefits might outweigh the harms. Outside this age range, PSA-based screening as a routine could not be recommended, based on the available evidence. For men aged <55 years at higher risk (e.g., with a positive family history), decisions about prostate cancer screening should be individualised [Citation28].
The increasing awareness of the general public towards prostate cancer is very important. Participation in this early-detection programme will depend mainly on the level of knowledge and quantity of information provided to the patient and their families. Such attitudes should rely on a solid background of proper information and motivation from physicians [Citation13].
Our study has some limitations, being retrospective and including relatively few patients; the small sample size probably reflects the low prevalence of prostatic cancer in Saudi Arabia.
Furthermore most of the patients were referred from another source that had the initial contact with the patients. A large multicentre study from different centres in Saudi Arabia is needed to give valid data about the real epidemiology of prostate cancer. We hope that the present study will provoke such larger multicentre studies.
In conclusion, in the light of the current low prostate cancer prevalence rate, a hospital-based early-detection programme appears to be a more appropriate measure to diagnose the disease at earlier curable stages, and thus improve the clinical outcome compared to a population-based programme. Further research should be on a larger scale and multicentred.
Conflict of interest
None.
Source of Funding
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- I.G.AlghamidiI.I.HussainM.S.AlghamdiM.A.El-SheemyThe incidence rate of prostate cancer in Saudi Arabia: an observational descriptive epidemiological analysis of data from the Saudi Cancer Registry 2001–2008Hematol Oncol Stem Cell TherS1658–38761320130007679
- S.A.TahaB.A.KamalScreening program for prostate cancer at a University hospital in Eastern Saudi ArabiaSaudi Med J26200511041106
- A.J.AbusamraS.BazarbashiY.BahaderH.KushiD.RabahN.Al Bogamiet alSaudi Oncology Society clinical management guidelines for prostate cancerUrol AnnSuppl.2011S106
- L.M.NewcomerJ.L.StanfordB.A.BlumensteinM.K.BrawerTemporal trends in rates of prostate cancer: declining incidence of advanced stage disease in 1974–94J Urol158199714271430
- A.L.MooreP.DimitropoulouA.LaneP.H.PowelD.C.GreenbergC.H.Brownet alPopulation-based prostate-specific antigen testing in the UK leads to a stage migration of prostate cancerBJU Int104200915921598
- G.AusS.BergdahlP.LoddingH.LiljaJ.HugossonProstate cancer screening decreases the absolute risk of being diagnosed with advanced prostate cancer – results from a prospective, population-based randomized controlled trialEur Urol512007659664
- F.H.SchröderJ.HugossonS.CarlssonT.TammelaL.MäättänenA.Auvinenet alScreening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC)Eur Urol622012745752
- H.A.MosliProstate cancer in Saudi Arabia. A review of the literature (1975–96)Ann Saudi Med171997510514
- J.S.KohC.W.ChengK.T.FooSpectrum of prostate cancer in the Singapore General Hospital (1980–85)Ann Acad Med Singapore302001513515
- P.D.EkwereS.N.EgbeThe changing pattern of prostate cancer in Nigerians: current status in the southeastern statesJ Natl Med Assoc942002619627
- M.MoketeD.C.ShackleyC.D.BettsK.J.O’FlynnN.W.ClarkeThe increased rate of prostate specific antigen testing has not affected prostate cancer presentation in an inner city population in the UKBJU Int972006266269
- E.ScosyrevE.M.MessingS.MohileD.GolijaninG.WuProstate cancer in the elderly. Frequency of advanced disease at presentation and disease-specific mortalityCancer118201230623070
- M.A.ArafaD.M.RabahI.H.WahdanAwareness of general public towards cancer prostate and screening practice in Arabic communities: a comparative multi-center studyAsian Pac J Cancer Prev13201243214326
- G.L.AndrioleE.D.CrawfordR.L.Grubb3rdS.S.BuysD.ChiaT.R.Churchet alPLCO Project Team. Mortality results from a randomized prostate-cancer screening trialN Engl J Med360200913101319
- F.H.SchröderJ.HugossonM.J.RoobolT.L.TammelaS.CiattoV.Nelenet alScreening and prostate-cancer mortality in a randomized European studyN Engl J Med360200913201328
- F.H.SchröderM.J.RoobolERSPC and PLCO prostate cancer screening studies: what are the differences?Eur Urol5820104652
- S.J.RabadiCancer at Dhahran Health Center, Saudi ArabiaAnn Saudi Med71987288293
- O.Z.Al-AbdinD.M.RabahA.AprikianDifferences in prostate cancer detection between Canadian and Saudi populationsBraz J Med Biol Res462013539545
- E.I.SalimM.A.MooreJ.A.Al-LawatiJ.Al-SayyadA.BazawirA.Beneret alCancer epidemiology and control in the arab world – past, present and futureAsian Pac J Cancer Prev102009316
- M.GhafoorR.SchuytenA.BenerEpidemiology of prostate cancer in United Arab EmiratesMed J Malaysia582003712716
- H.A.MosliT.A.Abdel-MeguidJ.A.Al-MaghrabiW.K.KamalH.A.SaadahH.M.FarsiThe clinicopathological patterns of prostatic diseases and prostate cancer in Saudi patientsSaudi Med J30200914391443
- I.A.HekalN.A.El-TabeyM.A.NabeehA.El-AssmyM.Abd El-HameedA.Nabeehet alValidation of Epstein criteria of insignificant prostate cancer in Middle East patientsInt Urol Nephrol422010667671
- R.KirbyCase study. Management of advanced prostate cancer with soft tissue metastasesProstate Cancer Prostatic Dis82005290292
- T.A.Abdel-MeguidH.A.MosliJ.A.Al-MaghrabiProstate inflammation. Association with benign prostatic hyperplasia and prostate cancerSaudi Med J30200915631567
- A.AlcarazP.HammererA.TubaroF.H.SchröderR.CastroIs there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature reviewEur Urol552009864873
- C.De NunzioG.KramerM.MarbergerR.MontironiW.NelsonF.Schröderet alThe controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammationEur Urol602011106117
- Y.NakaiN.NonomuraInflammation and prostate carcinogenesisInt J Urol202013150160
- H.B.CarterP.C.AlbertsenM.J.BarryR.EtzioniS.J.FreedlandK.L.Greeneet alEarly detection of prostate cancer: AUA GuidelineJ Urol1902013419426
- A.HeidenreichP.A.AbrahamssonW.ArtibaniJ.CattoF.MontorsiH.Van Poppelet alEarly detection of prostate cancer. The European Association of Urology recommendationsEur Urol642013347354