Abstract
Objective:
To determine whether the severity of erectile dysfunction (ED) in a man diagnosed with late-onset hypogonadism (LOH) gives information about his metabolic syndrome state, as patients with LOH often have sexual symptoms and associated cardiovascular and metabolic comorbidities, but the role of ED in predicting the prevalence of comorbid disease in men with low levels of testosterone is currently unknown.
Patients and methods:
Men (130) diagnosed with LOH and fulfilling the criteria of a total testosterone level of <3.5 ng/mL (<12 nmol/L), and with an erectile function domain score of <21 on the International Index of Erectile Function questionnaire (IIEF, questions 1–5), were enrolled for a subsequent trial of supplementation with testosterone undecanoate. Demographic data were recorded at baseline. The men completed three standardised questionnaires to assess sexual health, including the International Prostate Symptom Score, Ageing Males Symptoms (AMS) and IIEF Sexual Health Inventory for Men (SHIM). Patients were stratified by the severity of ED, with SHIM scores of 1–7 considered severe, 8–11 moderate, and 12–16 mild to moderate. Levels of serum testosterone, sex hormone binding globulin (SHBG) and lipids (total cholesterol, triglycerides, high-density and low-density lipoprotein) were assessed, along with plasma fasting glucose and glycated haemoglobin (HbA1c) levels. Body weight, body mass index and waist circumference were also recorded.
Results:
There was a significant association between the severity of ED and mean weight (P < 0.001), waist circumference (P < 0.001), triglycerides (P = 0.009), total cholesterol (P = 0.027), HbA1c (P < 0.001), fasting glucose (P = 0.003) and AMS scores (P = 0.043). There were no significant differences in testosterone fractions and SHBG levels between the ED subgroups. There was a positive correlation between the prevalence of diabetes mellitus (type 1 and type 2) and the severity of ED in these men (P = 0.018).
Conclusions:
The descriptive data showed that a greater severity of ED in men with LOH correlated with an increased waist circumference, hyperglycaemia, hypertriglyceridaemia, hyperlipidaemia, and a history of diabetes mellitus. Severe ED is a prognostic indicator of comorbidities in men with LOH.
Abbreviations:
- ED, erectile dysfunction
- LOH, late-onset hypogonadism
- IIEF, International Index of Erectile Function
- AMS, Ageing Males Symptoms
- SHIM, Sexual Health Inventory for Men
- SHBG, sex hormone-binding globulin
- HDL, high-density lipoprotein
- LDL, low-density lipoprotein
- HbA1c, glycated haemoglobin
- BMI, body mass index
- MetS, metabolic syndrome
- DM(1,2), diabetes mellitus (type 1, type 2)
- S, M, MM, severe, moderate, mild to moderate
- CVD, cardiovascular disease
Introduction
The definition of hypogonadism no longer remains ambiguous among practitioners, although the diagnosis of late-onset hypogonadism (LOH) is complicated by the syndrome’s non-specific symptoms. Whilst many acknowledge the role of testosterone in affecting erectile function, others believe that the decline in testosterone levels in men is not a primary causal factor for the decrease in sexual function. Rather it is a combination of many pathophysiological neural, vascular, hormonal and structural changes as sequelae of the ageing process that contribute to erectile dysfunction (ED). Even among experts who advocate that LOH is the main agent causing ED, a definitive agreement over the subphysiological ranges of testosterone has not been reached. However, recently the European Male Ageing Study group defined 3.20 mg/dL as the lower limit of the ‘normal’ total testosterone range in men, or a level of <220 pmol/L (64 pg/mL) as the lower limit of free testosterone, a definition that has been widely used and accepted [Citation1]. These values were recently revised by Bhasin et al. [Citation2] to establish 3.54–12.1 nmol/L as the normal range.
In addition, studies suggest strong correlations between low testosterone levels, obesity, and the metabolic syndrome (MetS) [Citation3]. Although the causality of this association is difficult to define, there is substantial evidence indicating that the relationship between low testosterone levels and components of MetS, including obesity and type 2 diabetes mellitus (DM2), is bidirectional, governed by a perpetuating cycle whereby worsening in one condition worsens the other [Citation3]. Concurrently, the presence and development of ED is often considered to be an early indicator of underlying cardiometabolic pathology due to direct correlations between the severity of ED and cardiovascular risk factors [Citation4,Citation5]. Given this picture we investigated whether the severity of ED in a man diagnosed with LOH gives information about his MetS state.
Patients and methods
This was a population-based observational study to assess comorbidities in men with LOH and varying degrees of ED, to establish relationships between these comorbidities and patients with LOH in general, and subgroups of LOH stratified by the severity of ED in particular. Between November 2004 and October 2011, men attending a urology clinic in Germany, and diagnosed with a total testosterone level of <3.5 ng/mL and scores of <21 on the erectile function domain (questions 1–5) of the International Index of Erectile Function (IIEF) were enroled in the study (reference range adopted from [Citation2]). In all, 130 men met the inclusion criteria.
Before starting the study we obtained approval from the ethics committee at the institution, in line with guidelines formulated by the German Arztekaumer (German medical association). Patients were enroled only after having signed an informed written consent, and the confidentiality of the data collected was ensured.
All patients were evaluated by a clinical assessment, with demographic data collected at baseline, consisting of their medical history, previous surgical history, medications, alcohol and tobacco usage, weight, height, body mass index (BMI) and waist circumference. Total testosterone, sex hormone-binding globulins (SHBG), thyroxine, total cholesterol, triglycerides, high-density lipoprotein (HDL) and low-density lipoprotein (LDL), plasma fasting glucose and glycosylated haemoglobin (HbA1c), glucose, and blood counts were assessed from blood samples taken in the early morning hours to minimise diurnal fluctuations. From total testosterone and SHBG levels, free and bioavailable testosterone values were calculated using the Vermeulen equation [Citation6]. Resting blood pressure was also assessed at baseline and averaged over the course of three measurements to reduce the potential effects of false readings. Prostate safety variables were also assessed, including PSA level, a DRE, prostate volume, postvoid residual urine volume and the IPSS questionnaire. Concomitantly, subjects completed standardised questionnaires to self-assess their sexual function, the ageing male’s syndrome (AMS), and a short version of the IIEF (IIEF-5) [Citation7]. Based on the responses, patients were classified into one of three categories of severity of ED, i.e., an IIEF-5 score of 1–7 was considered to be severe ED (S), 8–11 as moderate (M), and 12–16 as mild to moderate ED (MM).
The results were analysed statistically after stratification by severity of ED. A one-way ANOVA was used to compare the means of continuous variables between the severity groups, and the Pearson chi-squared analysis to compare between the proportions of categorical variables within the ED subgroups. Lastly, univariate and multivariate regression analyses were used to determine the predictors of severe ED in patients with diagnosed LOH. Statistical significance was indicated at P < 0.05.
Results
The mean (SD) age of the men was 65.2 (6.8) years. There were no statistically significant differences in age between the ED groups. The demographic variables are given in ; 21% of the men had cardiovascular disease (CVD), 34% had DM, 16.9% had hypertension, and 47.7% dyslipidaemia, with 46% smokers, 10% ex-smokers and 64% alcohol consumers. The mean (SD) levels of total testosterone, SHBG, free and bioavailable testosterone are also given in .
Table 1 The descriptive data for the men with LOH.
Comparing the means of the ED subgroups by one-way ANOVA there were no correlations with severity and any measure of the hormonal panel (serum total testosterone and SHBG, and calculated free and bioavailable testosterone) (). Of the continuous variables the one-way ANOVA showed that the means of body weight, waist circumference, fasting glucose, HbA1c, triglycerides and AMS were directly proportional to the severity of ED, with statistical significance ( and ).
Figure 1 Box-plot presentation of the independent variables with significant differences among the ED subgroups by One Way ANOVA analysis.
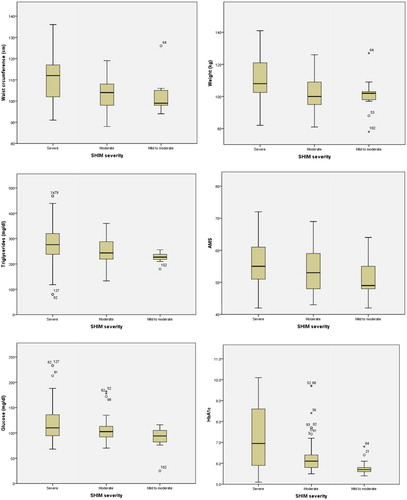
Table 2 A one-way ANOVA comparing the means of testosterone and SHBG levels between the ED severity subgroups.
Table 3 A one-way ANOVA comparing independent variables between the ED severity subgroups.
The distribution of DM showed that in the MM subgroup no patient had a positive history of DM. In the M subgroup 21.7% had DM2 and 4.3% had DM type 1 (DM1), and in the S subgroup 36.6% had DM2 and 8.5% had DM1. The prevalence of hypertension in the MM, M and S groups was 30.8%, 15.2%, and 15.5%, respectively. The prevalence of a history of LUTS was not significantly different between the ED groups, with 76.9%, 80.4% and 73.2% in the MM, M, and S subgroups, respectively.
On the univariate analysis, waist circumference, height, haemoglobin HbA1c, triglycerides, AMS, history of a CVD, DM, and smoking were statistically positive predictors of ED severity (). The multivariate analysis showed that waist circumference, fasting glucose, HbA1c, triglycerides, AMS, a history of CVD, DM and alcohol consumption were statistically positive predictors of ED severity ().
Table 4 Univariate and multivariate analysis for predictors of the severity of ED.
Discussion
This was an observational study of men presenting at our urology clinic for symptoms of sexual dysfunction and LOH. The present study shows that the severity of ED functions as a predictor of comorbidities in men with LOH. Of clinical importance, the evidence presented here supports the premise that ED might be a marker for underlying risk of CVD in hypogonadal men, and otherwise asymptomatic men, thus supporting a role for assessing the prevalence of additional disease in men with ED and low testosterone levels [Citation8].
It was previously postulated that ED is strongly related to components of MetS and cardiovascular risk. Feldman et al. [Citation9] found that DM2 is the second strongest predictor of ED after age. In the present study, among the comorbidities reported by the patients, a history of DM2 was associated with the severity of ED. ED is reported to be more common in men with DM2 and, furthermore, in 12–30% of men ED is the first sign of DM diagnosed later [Citation10]. A history of CVD showed a positive correlation with increased ED severity in the univariate and in the multivariate regression models. Nonetheless, the relationship was not as strong as the relationship of DM with ED, with higher P values in both the univariate (0.038 vs. 0.002 for CVD and DM, respectively) and the multivariate (0.023 vs. 0.003) analyses. This might be secondary to a confounding component in the predictive power of ED for CVD, due to an effect of DM2, especially in uncontrolled DM2, as CVD is a unanimous sequel. Also, when DM does not accompany CVD, ED presents later during the course of the illness. Waist circumference and triglyceride levels are two major markers of MetS, as indicated by the International Diabetes Federation [Citation11]. HbA1c is not used in their definition of MetS, but nevertheless it is a marker for diabetic control. In the present study, waist circumference, HbA1c and triglyceride levels were associated with the severity of ED in hypogonadal men in both the multivariate and univariate analysis. Ogden et al. [Citation12] and others [Citation13] showed that ED is more prevalent in men with the MetS, especially in obese patients. However, the scope of the present study does not include an assessment of whether the presence of ED is an effect of comorbidities or not, as all of the men were enroled on the basis of having ED at the start. In the assessment of social habits, the use of tobacco had positive predictive power of worse ED. However, the correlation lost its statistical significance in the multivariate analysis. This might be due to the confounding effect of CVD.
Whilst the mean age of the present men is comparable to that in other studies, it is apparent that these men had considerable comorbidities on the clinical assessment. This is predictably dictated by the inclusion criteria of LOH, whereby the age-related decline in testosterone levels is a central feature of LOH. Whilst advancing age is associated with increased prevalence of disease we conclude from the present study that the comorbidity profile of these men was directly related to the low testosterone levels in the men rather than due to the mere ageing process. By contrast, the attribution of ED as a result of hypogonadism remains debated. Marberger et al. [Citation14] refute the hypothesis that testosterone concentration is an indicator of ED, and state that age is a better predictor, and that this might also confound the capacity of testosterone level to predict concurrent disease. However, clinical and mechanistic studies indicate that androgens have a profound role in male sexual function, with testosterone regulating anatomy, penile vasculature and the response to vaso-active agents [Citation15,Citation16]. Indeed, about a third of men with ED have overt hypogonadism regardless of age [Citation17]. As such, a testosterone deficiency is considered to be a predisposing factor for ED [Citation18]. Others found that testosterone levels are inversely related to ED in animal models [Citation19], as well as human studies [Citation1,Citation17]. In the present study we were unable to detect a statistically significant difference in the severity of ED when comparing testosterone levels. We thus consider that, at least within our selected subjects, serum testosterone levels (total, calculated free and bioavailable) do not influence ED below the lower limit of the reference range. This is in agreement with Bhasin et al. [Citation2] who showed that the incidence of ED was statistically higher if testosterone levels were <3.5 ng/mL (12 nmol/L) and that below the subphysiological reference limit, the severity of ED does not increase. In addition, measured low testosterone levels in the blood are often not reproducible because of circadian and other variations, which might necessitate several measurements before reaching confirmatory levels.
Much evidence now supports a role for testosterone in health and disease, with low circulating levels associated with CVD, DM2, MetS and obesity, although this relationship might be independent of ED [Citation3]. Moreover, the correlation between hypogonadism and MetS is widely accepted [Citation3]. The causality of low testosterone levels and associated comorbidities is difficult to determine and a bidirectional relationship is likely. However, whilst the comorbidity profile of the present cohort was related to low testosterone levels, differences in serum testosterone level were no different between the stratified ED groups, but the incidence of cardiovascular and diabetic comorbidity was greater with increasing severity of ED. A direct comparison of testosterone level with the prevalence of comorbidity was beyond the scope of this report, although this has been shown in previous studies [Citation20].
A limitation to the present study is that correlational and regression analysis, univariate and multivariate, were used retrospectively, preventing a direct causal relationship to be ascertained from the data.
In conclusion, the aim of this study was to assess whether LOH is an ‘all-or-none’ disease, or whether subgroups within this population warrant more special attention. According to the present results there is a clear need to investigate the comorbid profile in patients with low levels of testosterone when they report severe ED or worsening of their ED. Reciprocally, we believe that when patients with known multiple comorbidities, e.g., DM, present to general clinics with complaints of ED, urologists and practitioners should investigate testosterone levels. Whether there is a role for testosterone replacement therapy in the treatment of ED, or improvement in the components of the MetS, remains debatable and is beyond the scope of this study. Nonetheless, we believe that reporting new-onset ED or worsening in the ED by patients known to have low testosterone levels should warn of a generally declining health condition, and warrants a reassessment of the management of the patient’s known and possible new comorbidities.
Conflict of interest
None.
Source of funding
None.
Acknowledgements
Editorial support for the manuscript was provided by Astra-Health, http://www.astra-health.co.uk.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- F.C.WuA.TajarJ.M.BeynonS.R.PyeA.J.SilmanJ.D.Finnet alIdentification of late-onset hypogonadism in middle-aged and elderly menN Engl J Med3632010123135
- S.BhasinM.PencinaG.K.JasujaT.G.TravisonA.CovielloE.Orwollet alReference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohortsJ Clin Endocrinol Metab96201124302439
- D.M.KellyT.H.JonesTestosterone. A metabolic hormone in health and diseaseJ Endocrinol2172013R25R45
- R.ShabsighM.A.PerelmanD.C.LockhartT.F.LueG.A.BroderickHealth issues of men: prevalence and correlates of erectile dysfunctionJ Urol1742005662667
- V.KupelianR.ShabsighA.B.AraujoA.B.O’DonnellJ.B.McKinlayErectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging StudyJ Urol1762006222226
- A.VermeulenL.VerdonckJ.M.KaufmanA critical evaluation of simple methods for the estimation of free testosterone in serumJ Clin Endocrinol Metab84199936663672
- R.C.RosenA.RileyG.WagnerI.H.OsterlohJ.KirkpatrickA.MishraThe international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunctionUrology491997822830
- G.JacksonJ.BetteridgeJ.DeanI.EardleyR.HallD.Holdrightet alA systematic approach to erectile dysfunction in the cardiovascular patient: a Consensus Statement – update 2002Int J Clin Pract562002663671
- H.A.FeldmanI.GoldsteinD.G.HatzichristouR.J.KraneJ.B.McKinlayImpotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging StudyJ Urol15119945461
- Z.A.A.KamenovComprehensive review of erectile dysfunction in men with diabetesExp Clin Endocrinol Diabetes1232015141158
- International Diabetes Federation. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf.
- C.L.OgdenM.D.CarrollL.R.CurtinM.A.McDowellC.J.TabakK.M.FlegalPrevalence of overweight and obesity in the United States, 1999–2004JAMA295200615491555
- K.ChitaleyV.KupelianL.SubakH.WessellsDiabetes, obesity and erectile dysfunction: field overview and research prioritiesJ Urol1826 Suppl.2009S45S50
- M.MarbergerT.H.WilsonR.S.RittmasterLow serum testosterone levels are poor predictors of sexual dysfunctionBJU Int1082011256262
- R.ShabsighThe effects of testosterone on the cavernous tissue and erectile functionWorld J Urol1519972126
- A.AversaA.M.IsidoriM.U.De MartinoM.CaprioE.FabbriniM.Rocchietti-Marchet alAndrogens and penile erection: evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunctionClin Endocrinol (Oxf)532000517522
- A.M.IsidoriE.GiannettaD.GianfrilliE.A.GrecoV.BonifacioA.Aversaet alEffects of testosterone on sexual function in men: results of a meta-analysisClin Endocrinol (Oxf)632005381394
- F.SaadA.S.GrahlA.AversaA.A.YassinA.KadiogluI.Moncadaet alEffects of testosterone on erectile function: implications for the therapy of erectile dysfunctionBJU Int992007988992
- A.M.TraishR.MunarrizL.O’ConnellS.ChoiS.W.KimN.N.Kimet alEffects of medical or surgical castration on erectile function in an animal modelJ Androl242003381387
- B.B.YeapZ.HydeO.P.AlmeidaP.E.NormanS.A.ChubbK.Jamroziket alLower testosterone levels predict incident stroke and transient ischemic attack in older menJ Clin Endocrinol Metab94200923532359
