Abstract
Many men have coexistent erectile dysfunction (ED) and lower urinary tract symptoms secondary to benign prostatic hyperplasia (LUTS/BPH). Phosphodiesterase type 5 (PDE5) inhibitors are effective for treating both of these conditions independently. In this review we summarise the evidence supporting a link between ED and LUTS/BPH, and the results from key clinical studies related to the use of PDE5 inhibitors for treating both conditions. The results from these studies suggest that men who have both ED and LUTS/BPH, and are concerned about their sexual dysfunction, might benefit from single-agent, holistic treatment with a PDE5 inhibitor.
Abbreviations:
- ED, erectile dysfunction
- QoL, quality of life
- 5ARI, 5α-reductase inhibitor
- PDE5, phosphodiesterase type 5
- NO, nitric oxide
- cGMP, cyclic guanosine monophosphate
- RhoA, Ras homologue gene family member A
- ROCK, Rho-associated protein kinase
- IIEF, International Index of Erectile Function
- EF, erectile function (domain)
- AE, adverse event
Introduction
Many men with LUTS secondary to BPH (LUTS/BPH) have coexistent erectile dysfunction (ED) [Citation1], suggesting that there might be a link between these conditions. Indeed, in a cohort of men scheduled for the surgical management of LUTS/BPH, 36% with moderate LUTS/BPH and 94% with severe LUTS/BPH were found to have coexisting ED [Citation2]. Further, in an integrated analysis of results from three clinical trials of tadalafil for LUTS/BPH, ≈70% of men had a history of ED [Citation3]. Interestingly, ED appears to be under-recognised by physicians treating men with LUTS/BPH. Specifically, the results of a survey in the USA showed that urologists estimate the prevalence of ED to be <20% in men with LUTS/BPH, and that primary-care physicians estimate the prevalence to be <30% [Citation4]. Hence, there appears to be a need for physicians to become more aware that a high proportion of men with LUTS/BPH also have ED. In addition to the well-known physical consequences of these conditions, both can have a pronounced negative effect on an individual’s quality of life (QoL), with men who have both ED and LUTS/BPH having more pronounced deficits in QoL than men who have either condition alone [Citation5]. As a result, many men with ED and/or LUTS/BPH will seek medical treatment.
The current medical treatments for LUTS/BPH include α-blockers, 5α-reductase inhibitors (5ARIs) and, more recently, the phosphodiesterase type 5 (PDE5) inhibitor, tadalafil. Of these, PDE5s are also a well-established and effective treatment for ED [Citation6]. Although α-blockers and 5ARIs can be effective for managing LUTS/BPH, these treatments can compromise sexual function in some men by decreasing libido, increasing the rate of ejaculatory disorders, and/or increasing the rate of ED [Citation7]. Specifically, in the CombAT [Citation8] and MTOPS [Citation9] randomised controlled trials, patients who received 5ARIs, α-blockers, or a combination of both therapies had rates of sexual adverse events of ≈1–9%, with the highest rate occurring with combined therapy [Citation7]. Furthermore, in a large retrospective study, Corona et al. [Citation10] found that the use of 5ARIs was associated with a higher risk of hypoactive sexual desire and a perceived reduction in sleep-related erections. Therefore, for men who have coexistent ED and LUTS/BPH, treatment with a PDE5 inhibitor might provide a holistic means of relieving the symptoms of both conditions without increasing the risk of sexual adverse events that might occur with α-blockers or 5ARIs.
The objective of this review was to summarise the evidence supporting a link between ED and LUTS/BPH, as well as evidence from key clinical studies on the use of PDE5 inhibitors for managing patients with ED and LUTS/BPH. In addition, we hope this review will serve to remind physicians that a large proportion of men with LUTS/BPH will also have ED, and vice versa. As tadalafil is the only PDE5 inhibitor currently approved for treating LUTS/BPH, our review focused on the findings from clinical studies of tadalafil.
Evidence supporting a link between ED and LUTS/BPH
Shared comorbidities
Consistent with a common underlying mechanism(s) and pathophysiology, LUTS/BPH and ED share several common risk factors, including age, diabetes mellitus, obesity, hypertension, and hypogonadism [Citation11–Citation13]. Of these, age is one of the most important risk factors for both ED and LUTS/BPH. However, evidence from population-based studies has consistently shown a correlation between LUTS/BPH and ED that is independent of age and other comorbidities, including diabetes mellitus and heart disease [Citation14]. These findings support the notion that there is an independent link between the conditions [Citation15].
Pathophysiology
Although not completely understood, several common pathophysiological mechanisms have been proposed to underlie both LUTS/BPH and ED, including reduced nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) signalling, increased Ras homologue gene family member A (RhoA)/Rho-associated protein kinase (ROCK) signalling, autonomic hyperactivity, and pelvic atherosclerosis. These mechanisms have been reviewed in great detail elsewhere [Citation14,Citation16,Citation17] and therefore will be only briefly discussed here.
The NO-cGMP pathway is important in smooth-muscle relaxation and erection of the penis [Citation14,Citation16,Citation17]. Likewise, NO inhibits smooth-muscle tone in the bladder, prostate, and urethra [Citation14,Citation16,Citation17]. Activation of the RhoA/ROCK signalling pathway decreases smooth-muscle relaxation tone in lower urinary tract tissues, including the prostate, bladder neck, urethra, and penis [Citation14,Citation16,Citation17]. Increased sympathetic nervous system activity might affect smooth muscle and vascular tone in the lower urinary tract via α1-adrenergic receptors in the penis, bladder, and prostate. Finally, atherosclerosis can result in decreased perfusion/ischaemia of lower urinary tract tissues [Citation14,Citation16,Citation17]. All of these pathophysiological changes are thought to contribute to the development of ED and LUTS/BPH [Citation14], and are consistent with a link between these conditions.
PDE5: location and function in the lower urinary tract
PDE5 isoenzymes are widely distributed throughout the body, including in the prostate, bladder, urethra, corpus cavernosum of the penis, and the lower urinary tract vasculature, specifically smooth muscle and endothelial cells [Citation16,Citation18]. Functionally, PDE5 isoenzymes are important in NO/cGMP signalling by catalysing the hydrolysis of cGMP [Citation19]. Inhibition of PDE5 isoenzymes is effective for relieving the symptoms of both ED and LUTS/BPH (see the following section). In the case of ED, inhibition of PDE5 leads to increased intracellular cGMP concentrations, which in turn results in smooth-muscle relaxation and increased blood flow to the corpus cavernosum [Citation19]. In the case of LUTS/BPH, PDE5 inhibition, and consequent alterations in NO/cGMP signalling, are thought to relieve symptoms by inducing the relaxation of prostatic smooth muscle and consequently reducing urethral pressure [Citation20].
Management of ED and LUTS/BPH with PDE5 inhibitors: findings from clinical studies
In this section we highlight the key findings from studies that have examined the use of PDE5 inhibitors for the on-demand management of ED in men with LUTS/BPH, ED and LUTS/BPH monotherapy, and the management of ED and LUTS/BPH in combination with the 5ARI, finasteride. The known safety and tolerability of PDE5 inhibitors are also summarised.
The management of ED in LUTS/BPH as separate entities: On-demand PDE5 inhibitor treatment
Until recently, a combination of α-blockers, on-demand PDE5 inhibitors, or other classes of drugs, were typically used and considered safe. However, PDE5 inhibitors should be used with caution in patients taking the α1-blockers doxazosin or terazosin [Citation21]. The objective of using on-demand PDE5 inhibitors is to treat ED independently of BPH symptoms. In an analysis of 11 double-blind, placebo-controlled trials on the efficacy of tadalafil, Lewis et al. [Citation22] showed that tadalafil 20 mg significantly improved erectile function in men with ED who had BPH as a comorbid condition. Specifically, the change from baseline in the least-squares mean International Index of Erectile Function (IIEF) [Citation23] erectile function (EF) domain scores was significantly more pronounced for men treated with tadalafil 20 mg than for men treated with placebo (8.4 vs. 1.1, P < 0.001; scores at the endpoint were 22.6 and 15.4, respectively). A higher proportion of men who were treated with tadalafil 20 mg attained a normal IIEF-EF score (<26) than men treated with placebo (48.6% vs. 11.3%, P < 0.001) [Citation22].
The management of ED and LUTS/BPH with PDE5 inhibitors as monotherapy
Three major PDE5 inhibitors have been studied in men with LUTS/BPH, i.e., tadalafil, sildenafil and vardenafil. A meta-analysis published by Gacci et al. [Citation24] combining the results of several trials of different PDE5 inhibitors showed that treatment with PDE5 inhibitors significantly improved the IIEF score (+5.5; P < 0.001) and the IPSS (−2.8; P < 0.001) compared with placebo.
Tadalafil has a half-life of 17.5 h, which allows for this specific PDE5 inhibitor to be taken once-daily to provide stable plasma concentrations. For LUTS/BPH, treatment with tadalafil has been reported to significantly decrease the IPSS by 22–37% [Citation21]. Furthermore, in several studies, tadalafil significantly improved the IPSS from baseline to endpoint compared with placebo, from 4.2 vs. −2.1, respectively (P = 0.004) [Citation25], and −6.3 vs. −4.2, respectively (P = 0.001) [Citation26]. The improvement in IPSS was reported to occur after 1 week of starting treatment with tadalafil [Citation21] and was shown to be maintained for up to 1 year [Citation27].
Tadalafil 5 mg once-daily improved erectile function regardless of age, LUTS/BPH severity, or previous ED therapy [Citation25]. In all of the studies that have been reviewed, men with signs and symptoms of BPH who were treated with tadalafil 5 mg once daily for 12 weeks had significant improvements in the total IPSS compared with placebo. In men who also had ED, administration of tadalafil 5 mg once daily led to an improvement in the IIEF erectile function domain score that was significantly better than that observed with placebo () [Citation25,Citation28]. Data from four randomised, placebo-controlled clinical studies of 1026 sexually active men with both LUTS/BPH and ED were pooled to evaluate the efficacy and safety of tadalafil 5 mg once daily for 12 weeks compared with placebo [Citation29]. The results of this analysis showed that the magnitude of improvement in total IPSS from baseline to endpoint was significantly greater for tadalafil than for placebo (mean change −6.0 vs. −3.6, P < 0.001). Likewise, the magnitude of improvement in the IIEF-EF score was significantly greater for tadalafil than for placebo (mean change 6.3 vs. 1.4, P < 0.001). As highlighted in , the improvement in the symptoms of ED occurred regardless of the baseline severity of LUTS/BPH [Citation29].
Table 1 The changes in IPSS and IIEF-EF scores from baseline to endpoint (in two studies [Citation25,Citation28] including men with both ED, and signs and symptoms of BPH; tadalafil 5 mg daily dose results). The efficacy analyses included only patients who had a baseline measurement and one or more measurements after baseline.
Table 2 The change in IIEF-EF score by baseline total IPSS category. Data from [Citation29].
Interestingly, in a randomised, placebo-controlled study of 606 men with both ED and LUTS/BPH, Egerdie et al. [Citation28] found that there was a significant improvement in ED (IIEF-EF) for tadalafil 2.5 mg or 5 mg compared with placebo, whereas there was a significant improvement in LUTS/BPH (IPSS) only for tadalafil 5 mg compared with placebo. The improvement in ED with tadalafil 2.5 mg and the lack of improvement in LUTS/BPH suggests that the therapeutic effect of tadalafil in ED and LUTS/BPH might be independent (i.e., the improvement in LUTS/BPH is not predominantly mediated by an improvement in ED). That men who do not have ED also have an improvement in LUTS/BPH with tadalafil 5 mg also supports this notion [Citation30].
Monotherapy with tamsulosin, a commonly prescribed α-blocker, or tadalafil has been found to result in significant and numerically similar improvements over placebo in the IPSS and maximum urinary flow rate in men with LUTS/BPH [Citation26]. By contrast, the improvement in ED was only seen in men treated with tadalafil [Citation26]. In another placebo- and tamsulosin-controlled study of sexually active men with ED and LUTS/BPH, tadalafil 5 mg once daily was found to maintain and improve sexual function, including ejaculation and orgasm, satisfaction, and EF (IIEF) compared with placebo [Citation31]. Men treated with tamsulosin 0.4 mg had no such improvements in sexual function, and indeed had lower ejaculation, orgasm, and overall satisfaction scores than men who received placebo [Citation31].
Management of ED and LUTS/BPH with PDE5 inhibitors in combination with finasteride
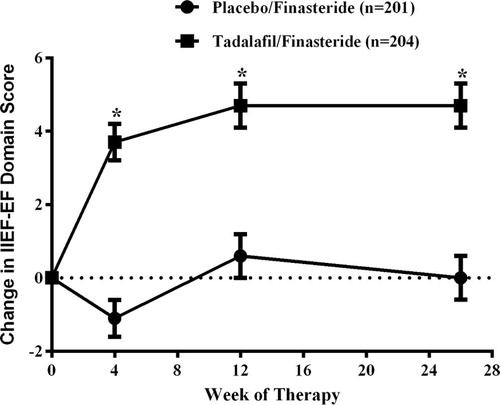
In men with moderate to severe LUTS and confirmed prostate enlargement, 5ARI monotherapy or combined with α-blockers is indicated to prevent BPH progression, reduce prostate size, and reduce the risk of urinary retention and future surgery [Citation32,Citation33]. Recently, an international, randomised, double-blind, parallel study in 695 men with LUTS/BPH assessed the efficacy and safety of the co-administration of tadalafil once daily with finasteride over a 6-month period [Citation32]. The combination provided an early improvement in LUTS and improved EF in men who had comorbid ED. The least-squares mean changes from baseline in the IIEF-EF were 3.7 after 4 weeks and 4.7 after 12 and 26 weeks of treatment with tadalafil and finasteride () [Citation32].
Figure 1 The change from baseline in IIEF-EF score over time in sexually active men with ED who were treated with placebo and finasteride, or placebo and tadalafil. Data are the least-squares mean (SEM). *Indicates a statistically significant difference between the groups (P < 0.001). Data from Casabé et al. [Citation32].
Safety and tolerability
The European Association of Urology guidelines [Citation21] and the Summary of Product Characteristics for tadalafil contain information on the safety of PDE5 inhibitors [Citation34]. Possible side-effects of PDE5 inhibitors include headache, back pain, dizziness, and dyspepsia. PDE5 inhibitors are contraindicated in men being treated with nitrates, potassium-channel openers, or nicorandil [Citation21]. Likewise, PDE5 inhibitors are contraindicated in men with myocardial insufficiency (New York Heart Association Stage >2), unstable angina pectoris, recent myocardial infarction (within 3 months), stroke (within 6 months), poorly controlled blood pressure, hypotension, significant hepatic insufficiency, or renal insufficiency. Anterior ischaemic optic neuropathy with sudden loss of vision has been reported after PDE5 inhibitor use [Citation21]. In addition, the co-administration of tadalafil (5 mg daily and 20 mg as a single dose) with doxazosin (4 and 8 mg daily) is known to significantly heighten the blood pressure-lowering effect of doxazosin, an effect which can last for ⩾12 h and be symptomatic (including syncope) [Citation34]. Therefore, this combination is not recommended [Citation34].
Results from clinical studies of LUTS/BPH have shown that the safety profile of tadalafil 5 mg is similar to the safety profile determined in clinical studies of tadalafil 5 mg once daily for treating ED [Citation35]. No unique safety issues have been identified in clinical studies of men with LUTS/BPH or in men with both LUTS/BPH and ED [Citation36]. The efficacy and safety of tadalafil 5 mg once daily for men with LUTS/BPH has been analysed by pooling data from four multinational, randomised, placebo-controlled clinical studies that included 1500 men [Citation35]. In the overall population, the proportion of patients with at least one adverse event (AE) was higher with tadalafil 5 mg (27.4%) than with placebo (20.9%) (P = 0.003) [Citation35]. The rate of treatment-emergent AEs was similar between subgroups for baseline age (⩽65 or > 65 years), previous PDE5 inhibitor use, and comorbidities (diabetes mellitus, hypertension, or cardiovascular disease), but were slightly higher for recent previous α-blocker use [Citation35].
In a 1-year open-label extension study of tadalafil 5 mg in men with LUTS/BPH, a total of 427 participants (mean age 63 years) were included and 299 (69.9%) completed the study. The most common reasons for early discontinuation included patient decision (59 men), adverse reaction (22), loss to follow-up (16), and lack of efficacy (15) [Citation27]. Of the 427 men, 57.6% reported at least one treatment-emergent AE. Dyspepsia (4.0%), gastro-oesophageal reflux disease (4.0%), back pain (3.7%), headache (3.0%), sinusitis (2.8%), hypertension (2.6%), and cough (2.1%) were the most common AEs. The mean (SD) PSA level increased from 1.6 (1.3) ng/mL at baseline to 1.8 (1.4) ng/mL at the study endpoint, whereas the mean postvoid residual urine volume decreased from 61.1 (60.4) mL at baseline study entry to 42.2 (64.1) mL at the study endpoint [Citation27].
summarises the AEs reported in two studies by men with ED and LUTS/BPH who were treated with tadalafil 5 mg once daily [Citation25,Citation28].
Table 3 Treatment-emergent AEs (reported in >2% of patients treated with tadalafil overall) in two studies [Citation25,Citation28] including men with both ED and signs and symptoms of BPH.
Conclusions
There is strong epidemiological and pathophysiological evidence supporting a link between ED and LUTS/BPH. From a clinical perspective, the apparent link between these two very common conditions suggests that a holistic approach to treatment might be effective for men who have both ED and LUTS/BPH. To this end, PDE5 inhibitors, which have long been used for treating ED alone, have more recently been found to be an effective treatment for LUTS/BPH. Indeed, the PDE5 inhibitor tadalafil is now approved in several countries for the treatment of LUTS/BPH. Furthermore, the 2013 European Association of Urology guidelines clearly state that ‘PDE5 inhibitors reduce moderate-to-severe (storage and voiding) LUTS in men with or without ED’ (level of evidence 1B and grade of recommendation A) and that ‘tadalafil can quickly reduce LUTS to a similar extent as α1-blockers and also improves ED’ [Citation21]. Therefore, men who have both ED and LUTS/BPH, and are concerned about their sexual dysfunction, might benefit from single-agent treatment with a PDE5 inhibitor.
Conflict of interest
Dr. Bulbul and Dr. Jabbour have served as paid consultants on a medical advisory board organised by Lilly on a related subject. Dr. Haddad was employed as a Medical Advisor for Eli Lilly when this manuscript was prepared.
Source of funding
None.
Acknowledgements
Medical writing assistance was provided by Luke Carey, PhD, and Serina Stretton, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2).
All authors participated in reviewing the literature, and in the drafting, critical revision, and approval of the final version of the manuscript.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- R.RosenJ.AltweinP.BoyleR.S.KirbyB.LukacsE.Meulemanet alLower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7)Eur Urol442003637649
- A.OzayarA.E.ZumrutbasO.YamanThe relationship between lower urinary tract symptoms (LUTS), diagnostic indicators of benign prostatic hyperplasia (BPH), and erectile dysfunction in patients with moderate to severely symptomatic BPHInt Urol Nephrol402008933939
- G.BrockG.BroderickC.G.RoehrbornL.XuD.WongL.ViktrupTadalafil once daily in the treatment of lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) in men without erectile dysfunctionBJU Int1122013990997
- A.SeftelR.RosenL.KuritzkyPhysician perceptions of sexual dysfunction related to benign prostatic hyperplasia (BPH) symptoms and sexual side effects related to BPH medicationsInt J Impot Res192007386392
- A.D.SeftelJ.de la RosetteJ.BirtV.PorterV.ZarotskyL.ViktrupCoexisting lower urinary tract symptoms and erectile dysfunction: a systematic review of epidemiological dataInt J Clin Pract6720133245
- K.HatzimouratidisE.AmarI.EardleyF.GiulianoD.HatzichristouF.Montorsiet alGuidelines on male sexual dysfunction: erectile dysfunction and premature ejaculationEur Urol572010804814
- V.MironeA.SessaF.GiulianoR.BergesM.KirbyI.MoncadaCurrent benign prostatic hyperplasia treatment. impact on sexual function and management of related sexual adverse eventsInt J Clin Pract65201110051013
- C.G.RoehrbornP.SiamiJ.BarkinR.DamiaoK.Major-WalkerI.Nandyet alThe effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT studyEur Urol572010123131
- J.D.McConnellC.G.RoehrbornO.M.BautistaG.L.AndrioleJrC.M.DixonJ.W.Kuseket alThe long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasiaN Engl J Med349200323872398
- G.CoronaG.RastrelliE.MaseroliG.BalerciaA.SforzaG.Fortiet alInhibitors of 5alpha-reductase-related side effects in patients seeking medical care for sexual dysfunctionJ Endocrinol Invest352012915920
- H.J.ParkJ.E.WonS.SorsaburuP.D.RiveraS.W.LeeUrinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) and LUTS/BPH with erectile dysfunction in Asian men: a systematic review focusing on tadalafilWorld J Mens Health312013193207
- J.K.ParsonsBenign prostatic hyperplasia and male lower urinary tract symptoms. Epidemiology and risk factorsCurr Bladder Dysfunct Report52010212218
- J.T.WeiE.CalhounS.J.JacobsenUrologic diseases in America project: benign prostatic hyperplasiaJ Urol173200512561261
- M.GacciI.EardleyF.GiulianoD.HatzichristouS.A.KaplanM.Maggiet alCritical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasiaEur Urol602011809825
- M.KirbyC.ChappleG.JacksonI.EardleyD.EdwardsG.Hackettet alErectile dysfunction and lower urinary tract symptoms: a consensus on the importance of co-diagnosisInt J Clin Pract672013606618
- K.E.AnderssonW.C.de GroatK.T.McVaryT.F.LueM.MaggiC.G.Roehrbornet alTadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of actionNeurourol Urodyn302011292301
- K.T.McVaryErectile dysfunction and lower urinary tract symptoms secondary to BPHEur Urol472005838845
- F.GiulianoS.UckertM.MaggiL.BirderJ.KisselL.ViktrupThe mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasiaEur Urol632013506516
- S.UckertM.A.KuczykM.OelkePhosphodiesterase inhibitors in clinical urologyExp Rev Clin Pharmacol62013323332
- P.WongN.LawrentschukD.M.BoltonPhosphodiesterase 5 inhibitors in the management of benign prostatic hyperplasia and erectile dysfunction: the best of both worldsCurr Opin Urol192009712
- M.OelkeA.BachmannA.DescazeaudM.EmbertonS.GravasM.C.Michelet alEAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstructionEur Urol642013118140
- R.W.LewisR.SadovskyI.EardleyM.O’LearyA.SeftelW.C.Wanget alThe efficacy of tadalafil in clinical populationsJ Sex Med22005517531
- R.C.RosenA.RileyG.WagnerI.H.OsterlohJ.KirkpatrickA.MishraThe International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunctionUrology491997822830
- M.GacciG.CoronaM.SalviL.VignozziK.T.McVaryS.A.Kaplanet alA systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with alpha-blockers for lower urinary tract symptoms due to benign prostatic hyperplasiaEur Urol6120129941003
- H.PorstK.T.McVaryF.MontorsiP.SutherlandA.Elion-MboussaA.M.Wolkaet alEffects of once-daily tadalafil on erectile function in men with erectile dysfunction and signs and symptoms of benign prostatic hyperplasiaEur Urol562009727735
- M.OelkeF.GiulianoV.MironeL.XuD.CoxL.ViktrupMonotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trialEur Urol612012917925
- C.F.DonatucciG.B.BrockE.R.GoldfischerP.J.PommervilleA.Elion-MboussaJ.D.Kisselet alTadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a 1-year, open-label extension studyBJU Int107201111101116
- R.B.EgerdieS.AuerbachC.G.RoehrbornP.CostaM.S.GarzaA.L.Esleret alTadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: results of a randomized, placebo-controlled, double-blind studyJ Sex Med92012271281
- H.PorstC.G.RoehrbornR.J.SecrestA.EslerL.ViktrupEffects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia and on erectile dysfunction in sexually active men with both conditions: analyses of pooled data from four randomized, placebo-controlled tadalafil clinical studiesJ Sex Med10201320442052
- G.A.BroderickG.B.BrockC.G.RoehrbornS.D.WattsA.Elion-MboussaL.ViktrupEffects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia in men with or without erectile dysfunctionUrology75201014521458
- F.GiulianoM.OelkeA.JungwirthK.HatzimouratidisS.WattsD.Coxet alTadalafil once daily improves ejaculatory function, erectile function, and sexual satisfaction in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia and erectile dysfunction: results from a randomized, placebo- and tamsulosin-controlled, 12-week double-blind studyJ Sex Med102013857865
- A.CasabeC.G.RoehrbornL.F.Da PozzoS.ZepedaR.J.HendersonS.Sorsaburuet alEfficacy and safety of the coadministration of tadalafil once daily with finasteride for 6 months in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasiaJ Urol1912014727733
- American Urological Association Practice Guidelines CommitteeAmerican Urological Association Guideline. Management of benign prostatic hyperplasia (BPH) 2003, updated 2006 and 20102010American Urological Association Education and Research, Inc. Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph [accessed 11 March 2015]
- Cialis [summary of product characteristics]. Eli Lilly. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000436/WC500026318.pdf [accessed 11 March 2015].
- H.PorstM.OelkeE.R.GoldfischerD.CoxS.WattsD.Deyet alEfficacy and safety of tadalafil 5 mg once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: subgroup analyses of pooled data from 4 multinational, randomized, placebo-controlled clinical studiesUrology822013667673
- European Medicines Agency. Assessment report: Cialis. European Medicines Agency. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000436/WC500137787.pdf [accessed 11 March 2015].
