?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective:
To evaluate the efficacy and safety of low-dose (45 mg) intravesical bacille Calmette–Guérin (BCG) therapy in the treatment of patients with non-muscle-invasive bladder cancer (NMIBC), as intravesical BCG is the most acceptable adjuvant therapy for NMI transitional cell carcinoma of the bladder. However, in the standard regimen, undesirable effects are the main cause of treatment discontinuation.
Patients and methods:
The present study included 37 men with primary NIMBC. All patients underwent complete TURB and 2 weeks later, a 6-week course of 45 mg BCG diluted in 50 mL isotonic saline was instilled into the bladder and retained for 2 h. Patients were evaluated for BCG efficacy (recurrence with or without progression) and safety by documentation of minor and/or major side-effects.
Results:
There were no major or severe side-effects and no treatment discontinuations. Local adverse effects occurred in 20 patients, while systemic effects, in the form of fever, occurred in six patients (16.2%). There was recurrence in 14 patients (37.8%) after 18–34 months, with disease progression (muscle invasion) in four (10.8%) after 6–18 months. The recurrence index was 0.39/100 patients/month and the mean (range) tumour-free period was 30.97 (7–36) months.
Conclusion:
Low-dose BCG intravesical therapy is an effective adjuvant treatment in NMIBC. However, this needs to be validated in future studies and in comparison with other proposed doses and/or regimens.
Introduction
Bladder urothelial cancer is the fourth most common malignancy diagnosed in American men [Citation1] and worldwide, it is the seventh most common malignancy in men and the seventeenth most common in women. It is estimated that, in 2002, ≈357,000 new cases of bladder cancer (BC) were diagnosed [Citation2].
At diagnosis, most of these cancers are non-muscle-invasive (NMI) lesions. These lesions have a high incidence of recurrence (up to 75%), which requires close monitoring and subsequent intervention [Citation3].
Transurethral resection of the bladder (TURB) is the initial and crucial step in managing NMIBC. The aim of TURB is to establish a histological diagnosis of the tumour; to determine the tumour stage (the pathologist must be able to evaluate the depth of tumour invasion), all clinically important prognostic factors, e.g. grade, number, size, and configuration of the tumour, as well as the presence of carcinoma in situ (CIS); and to completely remove all visible lesions [Citation4].
Intravesical instillation of BCG after initial TURB is intended to prevent tumour recurrence and progression [Citation5] and was first proposed by Morales et al. [Citation6] for the treatment of NMIBC. Since then, BCG therapy has been shown to be the most effective treatment in the prevention of recurrence and progression of NMIBC, especially for high-risk NMIBC [Citation7].
Despite BCG treatment, 30–50% of patients do not respond and ≈15% progress to muscle-invasive disease [Citation8,Citation9]. Various factors might explain the high percentage of BCG failures. First, full compliance with the current protocol is influenced by BCG-associated side-effects. Second, incorrect histological staging of tumours, due to high intra- and interobserver variability among pathologists, may also explain BCG failures [Citation10,Citation11]. Also, an incomplete tumour resection, reported in 20–62% of cases at restaging TURB, might underlie refractory disease [Citation12,Citation13].
Despite the widespread use of intravesical BCG instillation as a standard adjuvant therapy, there are still many questions needed to be answered, e.g., (i) the necessity of maintenance BCG therapy; (ii) the efficacy of low-dose BCG vs standard dose; and (iii) the superiority of combined therapy with BCG [Citation14,Citation15].
The present study was designed to evaluate the efficacy and safety of low-dose (45 mg) intravesical BCG therapy for treating patients with NMIBC.
Patients and methods
This was a prospective study following the tenets of the declaration of Helsinki and was approved by the Institutional Review Board. The present study was conducted in our Urology Department, between March 2010 and January 2014.
In all, 37 men with a mean (SD) age of 54 (5.6) years, with primary NMIBC with low- and intermediate-risk of recurrence and progression were included (; Ta, five patients; T1, 32; grade 2 in 25 patients and grade 3 in 12). Gross haematuria was the presenting symptom in 31 patients, five presented with recurrent UTI, and one was accidentally discovered during ultrasonography for an unrelated condition.
Table 1 Tumour characteristics.
The procedure commenced with a bimanual examination under anaesthesia, followed by a careful endoscopic examination of the entire urethra and bladder. The size (compared with the diameter of the resection loop), number, and location of tumours, as well as regions of erythema and mucosal abnormalities suggestive of CIS were identified and registered. Tumours were completely resected separately in fractions that included the exophytic part of the tumour, the underlying bladder wall with the detrusor muscle, and the edges of the resection area. The specimens from different fractions were referred to the pathologist in separate containers. At pathological examination bilharzial ova were discovered in the lamina propria in 10 patients (27%). Only one of the 37 patients (2.7%) had associated CIS.
At 2 weeks after TURB, in the absence of complications and with a sterile urine culture, a 6-week course of 45 mg BCG (Pasteur strain) diluted in 50 mL isotonic saline was instilled into the bladder and retained for 2 h [Citation16,Citation17].
Outcomes
Recurrence was defined as the appearance of a new tumour either of lower tumour stage and grade, or with the same pattern as the original tumour. Progression in tumour stage was defined by depth of bladder muscle invasion or by regional or distant metastasis. The recurrence index was calculated using the equation proposed by Pagano et al. [Citation18]:
The tumour-free period was considered as the time from TURB until the appearance of first tumour recurrence. BCG treatment-related side-effects were classified as minor (persistence of local symptoms and/or low-grade fever for <48 h) or major (severe or persistent local symptoms and/or higher-grade fever for >48 h).
The follow-up comprised urethrocystoscopy with bladder wash cytology every 3 months for 1.5 years (the end-point of the study). When recurrence occurred, a re-TURB was performed and the same BCG protocol was re-applied.
Results
For the safety of BCG, there were no major or severe side-effects and there were no delayed instillations or cessations of therapy. Local adverse effects occurred in 20 patients (54.1%; bladder irritability was reported in 10; dysuria in three, and microscopic haematuria in seven), while systemic effects, in the form of fever, occurred in six patients (16.2%).
All adverse effects were minor, self-limiting and responded to symptomatic treatment in the form of NSAIDs, antipyretics, and anticholinergics within 48 h after intravesical instillations ().
Table 2 Side-effects reported.
Recurrence occurred in 14 of the 37 patients (37.8%; ) and in 10 (27.0%) there was no disease progression after 18–34 months (two patients after 18 months; four after 24 months; two after 30 months; and two after 34 months). Seven of these patients had grade 2 tumours and three grade 3 tumours, which were treated by re-TURB followed by a second 6-week course of 45 mg BCG intravesically. Bilharzial ova were discovered in the specimens of three of these 10 patients. Recurrence with disease progression (muscle invasion) occurred in four patients (10.8%) after 6–18 months (one patient recurred after 6 months; two after 12 months; and one after 18 months). All four underwent surgery for grade 3 tumours, comprising radical cystectomy and urinary diversion. Bilharzial ova were discovered in three of the four patients. The recurrence index was 0.39/100 patients/month and the mean (range) tumour-free period was 30.97 (7–36) months.
Table 3 Characteristics of recurrence in the studied patients.
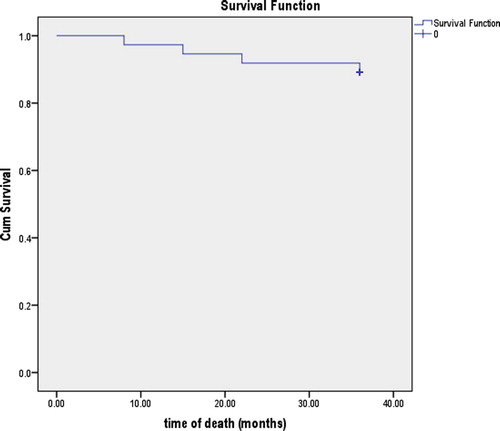
Four patients died (10.8%; death occurring at 8, 15, 22, and 36 months). The mean (SE; 95% CI) survival time was 34.29 (1.13; 32.08–36.51) months; the cumulative survival rate at 8 months was 97.3%; at 15 months 94.6%; at 22 months 91.9%; and at 36 months (end-point) 89.2% ().
Discussion
Since the introduction of BCG therapy by Morales et al. [Citation6], randomised trials have shown that BCG therapy reduces the frequency of tumour recurrences and prevents/delays stage progression better than TURB alone or topical chemotherapy [Citation19,Citation20]. Most practice guidelines recommend maintenance BCG for 1–3 years [Citation15,Citation21], while in others it is optional [Citation22]. However, maintenance BCG is associated with cumulative toxicity [Citation19]. In a Southwest Oncology Group (SWOG) trial, only 16% of patients were able to complete the 3-year maintenance schedule [Citation23]. Also, a review by Weizer et al. [Citation24] did not show significant differences in the cumulative incidence of recurrence or progression between patients receiving BCG alone or BCG with maintenance therapy. So, to determine our present patients’ compliance, we evaluated the efficacy and safety of low-dose (45 mg) intravesical BCG therapy for treating patients with NMIBC.
In the present study, recurrence occurred in 14 of the 37 patients (37.8%) and in 10 (27.0%) without disease progression after 18–34 months. In the remaining four patients (10.8%), recurrence with disease progression (muscle invasion) occurred after 6–18 months. There were no major or severe side-effects and there were no delayed instillations or cessations of therapy. Local adverse effects occurred in 20 patients (54.1%; bladder irritability was reported in 10; dysuria in three and microscopic haematuria in seven), while systemic effects, in the form of fever, occurred in six patients (16.2%). All adverse effects were minor, self-limiting, and responded to symptomatic treatment within 48 h of intravesical instillations. These results are comparable to those reported by Kamel et al. [Citation17] who reported that, nine patients (12.1%) had recurrence with muscle invasion (progression) after 6–18 months. Three of these nine patients had bilharzial ova in the specimen, while 19 patients (29.2%) had recurrent pT1 tumours after 16–45 months (seven with grade 3 tumours and 12 with grade 2). Five of these 19 patients had bilharzial ova in the lamina propria of the tumour. There were no major or severe side-effects and there were no delayed instillations or cessations of therapy. Local adverse effects occurred in 39 patients (52.7%), while systemic effects, in the form of fever, occurred in seven (9%). To summarise these results, BCG appeared to be effective and safe for NMIBC.
In two Egyptian studies, using the standard dose BCG regimen, Ismail et al. [Citation25] and Sarhan et al. [Citation26] reported that bladder irritability occurred in 90% and 52% of their patients respectively, severe enough in at least one-third of patients to result in discontinuation of therapy for 2–3 weeks, with administration of symptomatic treatment. They also reported that systemic sepsis occurred in 15% and 17% of patients respectively, in the form of high-grade fever (⩾39 °C), prostatic abscess, urinary tract tuberculosis, and epididymo-orchitis. With these severe and major adverse effects they administered anti-tuberculous treatment with permanent cessation of BCG therapy. Ismail et al. [Citation25] reported bladder contracture occurring in 3% of patients.
Herr et al. [Citation19] reviewed the long-term outcomes of a cohort of 1021 consecutive patients with high-risk NMIBC treated with induction BCG vs those treated with maintenance BCG. Overall, there were recurrences in 50% of patients at ⩽2 years, with muscle-invasive progression developing in 11%. Bladder cancer-specific death occurred in 4%. In all, 32% of the patients required another course of BCG therapy after induction.
In the SWOG 8507 trial, 550 patients with NMIBC with CIS or an increased risk of recurrence (defined as ⩾2 episodes of tumour within the most recent year, or ⩾3 tumours at ⩽6 months) were randomised to BCG induction alone or BCG induction plus 3-week maintenance given at 3, 6, 12, 18, 24, 30, and 36 months. At 3 months, 384 patients were disease free and thus eligible for comparison. Maintenance BCG significantly improved median recurrence-free survival (from 36 to 77 months; P < 0.001) at a median follow-up of 90 months, and worsening-free survival (defined as no evidence of progression including pathological stage ⩾T2 disease, or the use of cystectomy, systemic chemotherapy, or radiation therapy; P < 0.04) compared with BCG induction alone. Despite the increased use of cystectomy, radiation therapy, and systemic chemotherapy in the induction-only group, the 5-year survival increased from 78% to 83% with BCG maintenance (P = 0.08) [Citation27].
In 1991, the results of a randomised study evaluating the effectiveness and toxicity of a 75 mg Pasteur strain BCG (half the standard dose) for treating NMIBC were reported. The conclusion was that half of the standard BCG dose was as effective as adjuvant treatment against recurrent superficial papillary tumours. Complete response rates were similar to those achieved using the standard dose. Furthermore, treatment-related toxicity appeared to be significantly lower in patients with the low-dose regimen compared with the standard dose [Citation18].
In 1995, a phase II study evaluated the feasibility, response, and toxicity of 27 mg BCG (one-third the standard dose of the Connaught strain) in patients with high-risk NMIBC. The results of the study suggested that this low dose could be successfully used as adjuvant therapy for high-risk tumours with comparable results to the standard dose. Toxicity included local reactions, e.g. irritative bladder symptoms and haematuria, which were significantly lower than other reports using the standard dose [Citation28].
For toxicity, Pagano et al. [Citation18] reported that side-effects were significantly reduced with low-dose BCG therapy, in the form of bladder irritative symptoms in 27% and fever in 17% of patients. The Spanish Oncology Group (Club Urologico Espanol de Tratamiento Oncologico, CUETO) in 2007 [Citation23], reported minor local toxicity in 56% in the BCG-27 mg group and 51% in the BCG-13.5 mg group. Fever was reported in 7% in the BCG-27 mg group and 6% in the BCG-13.5 mg group [Citation23].
The present study has several limitations. Primarily, it included a small sample size, and Ta and T1 tumours were not studied separately. Also, a longer follow-up is needed.
In conclusion, low-dose BCG intravesical therapy is an effective adjuvant treatment in NMIBC. However, further studies are needed to validate and compare low-dose intravesical BCG therapy with other proposed doses and/or regimens.
Conflicts of interest
None declared.
Source of funding
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- A.JemalR.SiegelJ.XuE.WardCancer statistics, 2010CA Cancer J Clin602010277300
- M.GrassoBladder cancer: a major public health issueEur Urol Suppl72008510515
- H.NourO.AbdelrazakM.WishahyS.ElkatibH.BottoProstate-sparing cystectomy: potential functional advantages and objective oncological risks; a case series and reviewArab J Urol92011107112
- M.BabjukTransurethral resection of non-muscle-invasive bladder cancerEur Urol Suppl82009542548
- Z.XiaoE.HanelA.MakR.B.MooreAntitumor efficacy of intravesical BCG, gemcitabine, interferon-α and interleukin-2 as mono- or combination-therapy for bladder cancer in an orthotopic tumor modelClin. Med. Insights: Oncol.52011315323
- A.MoralesD.EidingerA.W.BruceIntracavitary bacillus Calmette–Guerin in the treatment of superficial bladder tumorsJ Urol1161976180183
- M.BabjukW.OosterlinckR.SylvesterE.KaasinenA.BohleJ.Palou-Redortaet alEAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 updateEur Urol5920119971008
- H.W.HerrA.MoralesHistory of bacillus Calmette–Guerin and bladder cancer: an immunotherapy success storyJ Urol17920085356
- J.W.DavisS.I.ShethM.J.DoviakP.F.SchellhammerSuperficial bladder carcinoma treated with bacillus Calmette–Guérin: progression-free and disease specific survival with minimum 10-year follow upJ Urol1672002494501
- M.H.AliI.Y.IsmailA.EltobgyA.GobeishEvaluation of second-look transurethral resection in restaging of patients with non-muscle invasive bladder cancerJ Endourol24201020472050
- B.W.Van RhijnT.H.Van der KwastD.M.KakiashviliN.E.FleshnerM.N.van der AaS.Alkhateebet alPathological stage review is indicated in primary pT1 bladder cancerBJU Int1062010206211
- M.AydinZ.TandogduF.O.KurtulusE.AvciA.FazliogluM.CekA prospective evaluation of second transurethral resection in non-muscle invasive bladder tumorsJ BUON152010514517
- R.T.DivrikA.F.SahinU.YildirimM.AltokF.ZorluImpact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trialEur Urol582010185190
- S.ZhuY.TangK.LiZ.ShangN.JiangX.Nianet alOptimal schedule of bacillus Calmette–Guerin for non-muscle-invasive bladder cancer: a meta-analysis of comparative studiesBMC Cancer132013332
- M.BabjukW.OosterlinckR.SylvesterE.KaasinenA.BöhleJ.Palou-RedortaEAU guidelines on non-muscle-invasive urothelial carcinoma of the bladderEur Urol542008303314
- M.BrausiL.ColletteK.KurthA.P.van der MeijdenW.OosterlinckJ.A.Witjeset alVariability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studiesEur Urol412002523531
- A.I.KamelA.G.El BazW.T.Abdel SalamM.E.RyadA.A.MahenaLow dose BCG regimen in T1 transitional cell carcinoma of the bladder: long term resultsJ Egypt Natl Canc Inst212009151155
- F.PaganoP.BassiC.MilaniA.MeneghiniD.MaruzziA.GarbeglioA low dose bacillus Calmette–Guerin regimen in superficial bladder cancer therapy: is it effective?J Urol14619913235
- H.W.HerrG.DalbagniS.M.DonatBacillus Calmette–Guérin without maintenance therapy for high-risk non-muscle-invasive bladder cancerEur Urol6020113236
- P.U.MalmströmH.WijkströmC.LundholmK.WesterC.BuschB.J.Norlén5-year follow-up of a randomized prospective study comparing mitomycin C and BCG in patients with superficial bladder carcinomaJ Urol161199911241127
- M.C.HallS.S.ChangG.DalbagniR.S.PruthiJ.D.SeigneSkinnerGuideline for the management of nonmuscle invasive bladder cancer [stages Ta, T1 and Tis]: 2007 updateJ Urol178200723142330
- J.E.MontieP.E.ClarkM.A.Eisenbergeret alNational Comprehensive Cancer NetworkBladder cancerJ Natl Compr Canc Netw72009839
- A.OjeaJ.L.NogueiraE.SolsonaN.FloresM.F.GómezJ.R.Molinaet alA multicentre, randomized prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette–Guerin [27 mg] versus very low-dose bacillus Calmette–Guerin [13.5 mg] versus mitomycin CEur Urol52200713981406
- A.Z.WeizerC.TallmanJ.S.MontgomeryLong-term outcomes of intravesical therapy for non-muscle invasive bladder cancerWorld J Urol2920115971
- M.H.IsmailM.M.WishahiA.AzizE.El ShimyA.KamelM.OsmanComplications of BCG immunotherapy for superficial bladder cancer: clinical comparison between Pasteur and Danish BCGMed J Cairo Univ641996119122
- O.SarhanB.Ali el DeinH.I.IbrahimM.A.GhoneimComplications of intravesical BCG therapy in superficial bladder tumors: a single centre studyEgypt J Urol8Suppl.200134 (abst.)
- D.L.LammB.A.BlunemsteinJ.D.CrissmanJ.E.MontieJ.E.GottesmanB.A.Loweet alMaintenance BCG immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group StudyJ Urol163200011241129
- D.MackJ.FrickFive-year results of a phase II study with low-dose bacille Calmette–Guerin therapy in high-risk superficial bladder cancerUrology451995958961