?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: To evaluate the impact of a luteinising hormone-releasing hormone (LHRH) agonist, goserelin acetate (GA), on surgical blood loss during transurethral resection of the prostate (TURP), as well as its histopathological effect on prostatic microvessel density (MVD).
Patients and methods: Patients who underwent TURP due to benign prostatic enlargement (60–100 mL) were randomly subdivided into two equal groups according to whether they received preoperative GA administration (3.6 mg; group A) or not (group B). Evaluation parameters were operative time, weight of resected prostatic tissue, perioperative haematocrit (HCT) changes, estimation of intraoperative blood loss, and suburethral and stromal prostatic MVD. Effects of GA on prostate weight and any possible side-effects were also monitored.
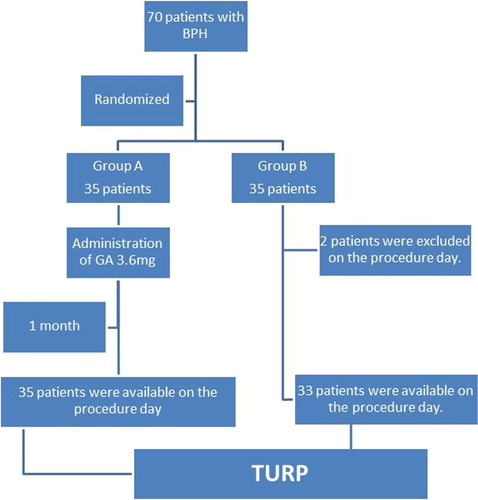
Results: In all, 35 and 33 patients were included in groups A and B, respectively. Operative time and HCT values’ changes were significantly less in group A (P < 0.05). Also, operative blood loss (both total and adjusted per weight of resected tissue) was lower in group A, at a mean (SD) of 178.13 (77.71) mL and 3.74 (1.52) mL/g vs 371.75 (91.09) mL and 8.59 (2.42) mL/g (P < 0.001). The median MVD in both suburethral [8 vs 11 vessels/high-power field (HPF)] and stromal tissues (9 vs 17 vessels/HPF) were significantly lower in group A (P < 0.001). Side-effects were minimal.
Conclusion: A single dose of GA, a LHRH agonist, before TURP is safe and effective in reducing surgical blood loss. It significantly reduced MVD in both suburethral and stromal nodular prostatic tissues without regional discrepancy.
Keywords:
Introduction
TURP is the ‘gold standard’ technique for managing BPH. Even within the era of lasers and bipolar vapourisation systems, monopolar TURP is still widely used, especially in developing countries. Despite marked improvements in TURP techniques, bleeding is still one of the undesirable perioperative events, with estimated transfusion rates ranging from 2% to 11% [Citation1].
To provide a strategy for decreasing blood loss, many researchers have focused on the natural history and pathophysiology of BPH-associated bleeding. Abnormal angiogenesis is considered to be the cause of BPH-related haemorrhage. Also, testosterone stimulates the release of growth factors, such as vascular endothelial growth factor (VEGF), resulting in increased microvessel density (MVD) and prostatic blood flow, whilst administration of anti-androgens in animal models leads to decreased prostatic blood flow and reduced angiogenesis [Citation2].
In humans, it has been found that using 5α-reductase inhibitors (5ARIs) leads to decreased prostatic bleeding in patients with BPH and those undergoing BPH-related surgery. A reduction in suburethral prostatic MVD (MVD-sub) has been suggested as the mechanism of action [Citation3]. Compared with 5ARIs, which mainly target dihydrotestosterone (DHT), LHRH agonists can produce a complete inhibition of testicular androgens (medical castration) [Citation4].
To our knowledge, there are no previous studies evaluating the effect of pre-treatment with a potent anti-androgen (LHRH agonist) on TURP-related blood loss. In the present study, we present the results of using goserelin acetate (GA) before TURP and its impact on surgical blood loss, as well as MVD of the prostate as a possible mechanism of action.
Patients and methods
Patients with symptomatic benign prostatic enlargement (BPE) planned for TURP were included in this randomised controlled clinical trial from January 2010 to June 2014. Included patients were those refractory to medical treatment with moderately enlarged prostates (60–100 mL). The exclusion criteria were patients with an abnormal coagulation profile, hepatic or renal impairment, abnormal DRE or high PSA (free/total ratio <0.25 or PSA density >0.15 ng/mL/mL), recent UTI, previous prostatic surgery, urethral catheterisation before surgery, neurogenic urinary bladder, and clinically evident non-BPE-related haematuria (e.g. urinary stones or urological tumours). The study protocol was reviewed and approved by our local Institutional Review Board.
The initial evaluation included a complete history, IPSS, clinical examination, uroflowmetry. Laboratory assessment included urine analysis, coagulation profile, complete blood count (CBC), haematocrit (HCT) values, liver and renal function tests, and serum PSA and testosterone levels. All patients underwent abdominal ultrasonography and TRUS (done by the same team of expert ultrasonographers), whilst further imaging of the upper urinary tract was performed when indicated.
Informed written consent was obtained from all participants, who were randomised into two groups according to whether they received a single s.c. injection of GA (3.6 mg) 4 weeks before TURP (group A) or not (group B). Randomisation was based on patients’ withdrawal of shuffled cards. For group A patients; IPSS, CBC, HCT, serum testosterone, TRUS measured prostate volume (TRUS-PV), and any drug-related side-effects were re-evaluated the day before TURP and taken as baseline values.
All patients underwent Mauermeyer monopolar TURP under spinal anaesthesia by the same surgeons who were ‘blinded’ to the patients’ groups. The volume and blood content of effluxed irrigant fluid (glycine 1.5%) was calculated for every patient. Perioperative i.v. hydration was maintained at 1500–2000 mL for all patients at a rate of 120–150 mL/h. Normal saline was used for postoperative bladder irrigation.
Intra-/post-procedure assessment included resection time, weight of resected tissues (using electronic scale) and changes in HCT (before and 48 h after procedures). Haemoglobin content of collected heparinised irrigant fluid was measured using HemoCue hemoglobinometer. Both total blood loss in irrigant fluid and adjusted blood loss/gramme of resected tissue (total blood loss in mL/weight of resected tissues in grammes) was estimated for every patient [Citation5].
Patients were discharged after satisfactory voiding of clear urine after catheter removal, whilst for those with unsatisfactory voids with significant residual urine (>100 mL), re-insertion of a catheter was performed for 3 days with follow-up.
MVD staining: The resected specimens from the prostatic urethra and remaining tissue were collected in separate paraffin-embedded blocks to be stained for histological and immunohistochemical studies. Haematoxylin and eosin (H&E) staining was performed to exclude malignancy and define epithelial/fibromuscular ratio. Whilst for immunohistochemical staining monoclonal CD34 antibodies were used, as this is a well-known immunohistochemical stain for prostatic MVD. Determination of MVD in both MVD-sub and hyperplastic nodular tissues (MVD-stroma) of the prostate, using a 10 × 10 reticulated imprinted grid at ×400, and expressed as the mean number of positively stained opened blood vessels with CD34-antibodies seen on 10 consecutive, non-overlapping high-power fields (HPF).
Statistics: The sample size was prospectively evaluated using G*POWER v3.1.9.2 software (Universitat Kiel, Germany), with effect size convention of 0.62, α error protection of 0.05 and power of 0.80. Based on these data, at least 33 patients had to be included in both study arms. Data were organised as mean ± SD using Statistical Package for Social Sciences for Windows (SPSS, Chicago, IL, USA). Student’s t-test (paired or unpaired) was used to compare different patient parameters with significant differences considered at P < 0.05.
Results
In all, 70 patients with symptomatic BPE were included and equally distributed in both groups. In group B, two patients were excluded on the day of TURP (one patient travelled abroad and the other refused TURP) (). Preoperative patients’ data were matched in both groups for age, IPSS, maximum urinary flow rate, serum PSA and testosterone levels, HCT, and TRUS-PV, and all had a pathological diagnosis of BPH. The number of patients receiving previous treatment in the form of 5ARIs was comparable in both groups (28 and 23 patients in groups A and B), with no statistically significant difference.
For group A patients, there were no preoperative statistically significant changes during the 4 weeks after GA pretreatment for the IPSS, at a mean (SD) of 24.73 (3.18) at presentation vs 24.92 (3.2) after treatment. Also, the mean (SD) haemoglobin, HCT and TRUS-PV were 12.8 (1.8) g/dL, 42.07 (1.98)% and 86.89 (9.96) mL, respectively at presentation vs 12.9 (1.5) g/dL, 41.8 (1.8)% and 85.83 (9.56) mL respectively just before TURP, these changes were not statistically significant. The encountered side-effects of GA were sexual dysfunction (nine patients), hot flushes (two), increased IPSS by 2 points (one) and diarrhoea (one).
Resected tissue weights were significantly higher in group A, at a mean (SD) of 50.54 (5.63) vs 43.90 (5.97) g (P < 0.001; ), whilst, the resection time was significantly lower in group A compared with group B (P < 0.001). Also, the postoperative data and changes in HCT values were significantly lower in group A compared with group B (P < 0.001).
Table 1 Perioperative data of patients in group A (GA-treated) and group B (controls).
During the postoperative period, bleeding was recorded in three patients in group B, which was controlled conservatively (bladder irrigation, tranexamic acid injection and blood transfusion of 1 unit), with a delay in catheter removal until the urine became clear. Satisfactory urinary voiding was achieved in all patients after catheter removal except in two patients in either group, a problem that was solved by re-catheterisation for 3 days.
For intraoperative blood loss; group A patients had significantly lower blood loss than group B (P < 0.001). Also, the adjusted blood loss per gramme of resected tissue was statistically significant lower in group A (P < 0.001). Histopathological examination confirmed the absence of malignancy in both groups.
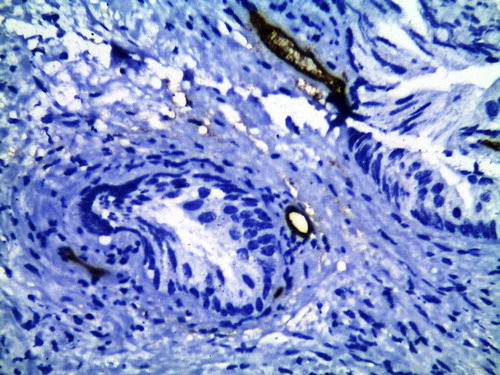
For MVD, the mean MVD-stroma was significantly lower in the GA-treated group compared with group B, at 8.46 (2.86) vs 16.79 (2.46) vessels/HPF (P < 0.001; and ). This significant reduction was also seen for MVD-sub in group A compared with group B, at 8.06 (2.94) vs 12.18 (3.3) vessels/HPF (P < 0.001; and ).
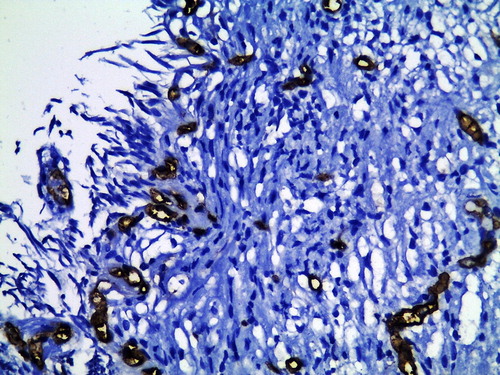
Figure 2 CD34 immunohistochemical staining of TURP section shows increased MVD-stroma in a control patient (×400).
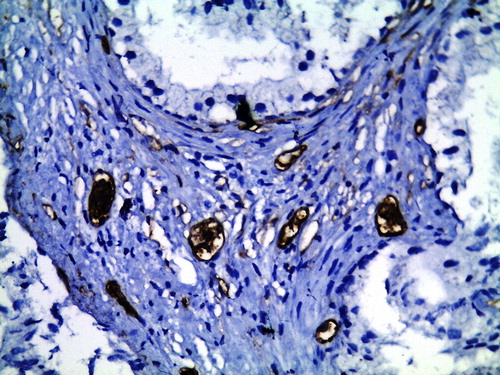
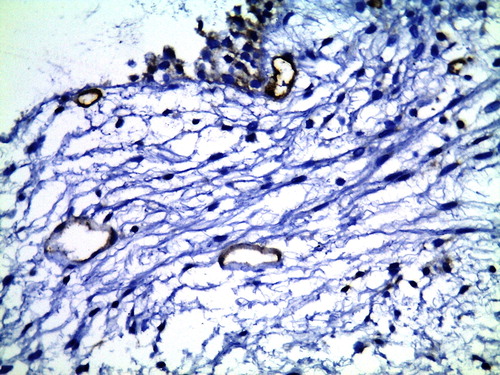
Figure 3 CD34 immunohistochemical staining of TURP section shows decreased MVD-stroma in GA-treated patient (×400).
Discussion
The concept of suppressed angiogenesis secondary to androgen deprivation was first described for controlling bleeding in patients with prostate cancer [Citation6], followed by studies of a similar association of finasteride (type II 5ARI) with BPH-associated haematuria. Meanwhile, the first evaluation of finasteride use before TURP was reported in 2000 by Hagerty et al. [Citation7]. These clinical studies were supported by experimental (human and animal) evidence showing the dose-dependent effects of androgens on hypoxia inducible factor 1α, expression of VEGF receptors, and other angiogenic pathways [Citation8]. Unlike prostatic adenomatous tissue, which is more sensitive to DHT, both testosterone and DHT were recognised to have a potent role in angiogenesis through expression of VEGF, which is an important regulator for pathological angiogenesis. It has been shown that suburethral prostatic acini have more VEGF expression than other prostatic stromal cells. In addition, anti-androgens were found to decrease MVD and blood flow within the prostate [Citation2,Citation9].
Further studies supporting the use of anti-androgens to reduce surgical blood loss (during TURP) attributed this effect to the ability to reduce angiogenesis [Citation10]. Prostatic MVD is frequently used as a histological and clinical indicator of angiogenesis. It has been shown that BPH tissues have higher vessel densities than normal prostatic tissues, especially in areas with nodular morphology. Also, patients with clinical BPH and recurrent gross haematuria have a significantly higher MVD-sub than patients with BPH alone [Citation11].
The available data suggest that MVD-sub would be reduced in patients treated with finasteride for 6 weeks, whilst no significant changes in MVD of the nodular hyperplastic areas occurred [Citation2,Citation12]. For dutasteride, which in theory could affect MVD as well as finasteride, the anti-angiogenic effect was more controversial with many studies denying this role [Citation13,Citation14].
For patients undergoing TURP, Ozdal et al. [Citation15] found that total blood loss was significantly reduced in the finasteride-treated group compared with the control group, irrespective of prostate volume. Furthermore, dutasteride (which is a type I and II 5ARI), some controlled trials confirmed its effectiveness in reducing prostate tissue vascularity in the peri-urethral area proximal to the verumontanum; improving operative time and reducing surgical blood loss [Citation9].
Unfortunately, 5ARIs can reduce only 70–94% of serum DHT and 85–100% of intraprostatic DHT without effect on other androgens [Citation16]. On the other hand, GA a ‘synthetic LHRH agonist’ can reduce testosterone and DHT to near castrate levels within 8–21 days after administration [Citation17] and this effect gradually diminishes over 3 months [Citation18]. Therefore, it could have a more potent role in decreasing intraoperative blood loss with a similar mechanism of action, i.e. reduction in MVD with an effective duration related to the administrated dose. In the present study, we report our experience with an early phase clinical trial with a small number patients for off-label use of a single GA dose given a month preoperatively, aiming at decreasing surgical blood loss during TURP.
Compared with previous 5ARIs reports, our present results showed that GA could significantly reduce MVD in both suburethral and hyperplastic nodular prostatic tissue. According to the Hochberg et al. [Citation12] series, the anti-angiogenic impact of finasteride was less apparent at the level of hyperplastic prostatic tissues for unknown reasons. They attributed this regional difference to the incompletely understood effects of finasteride on other BPH angiogenic pathways. In contrast, GA, which blocks most androgens forms, showed a significant MVD reduction even in hyperplastic prostatic tissues with the suggestion that other androgens, rather than DHT, have a possible role in the process of angiogenesis; but further studies are still warranted.
In our present patients, the significant reduction in MVD in both suburethral and hyperplastic prostatic tissues after GA injection was reflected by the reduction in surgical blood loss. We adopted four clinical parameters (operative time, HCT values, and both total and adjusted blood loss) to assess TURP associated bleeding. Also, the significantly more resected tissue weight in group A compared with group B patients may be explained by a clearer operative field due to minimal bleeding, allowing more resection in less operative time with less blood loss.
In the present study, the HCT value was used as an indicator for surgical blood loss. The changes in HCT values were significantly higher in group B. Practically; multiple factors could affect HCT changes, even with standardisation of perioperative hydration. Therefore, we calculated the total operative blood loss, which confirmed less blood loss in the patients injected with GA. This effect was more pronounced when adjusted for resected tissue weight. The reduction in resection time as well as the amount of blood loss is of utmost importance when used in patients with other comorbidities related to bleeding.
For pre-treatment duration, some authors found that finasteride could significantly reduce intraoperative blood loss if used from 4 to 12 weeks before TURP [Citation15,Citation19]. Similar results were reported by Pastore et al. [Citation20], when dutasteride was used for 6 weeks before treatment. Unfortunately, the later study lacked histological evidence to document this effect. Donohue et al. [Citation9] suggested a rapid anti-angiogenic effect of finasteride within 2 weeks; however, these data about the time of effect were not supported in further publications. Compared with those reports, in our present study, GA pretreatment only 4 weeks before TURP was enough to significantly reduce operative blood loss, with a significant reduction in both MVD-sub and MVD-stroma. Moreover, the use of GA as a single injection had a minimal and non-significant effect on prostate volume. According to Lukkarinen et al. [Citation21], a reduction in prostate volume may require up to 6 months of treatment with an LHRH agonist.
We excluded patients with huge prostates and those with conditions that may be associated with increased prostatic vascular density, e.g. concomitant bladder irritation by stones, prolonged indwelling catheter or UTI. Also, as a pilot study, we excluded ‘risky’ patients with bleeding disorders, although they could at least theoretically derive more benefit from decreased MVD and blood loss. However, further future trials are needed for validation of such a strategy.
In our present study, GA adverse reactions were of interest. All encountered side-effects were tolerable and only four patients (11.4%) reported non-sexual side-effects. In fact the main aspect of initial androgen flare after LHRH agonist therapy is only related to patients with prostate cancer and there are no published studies documenting its clinical impact on patients with BPH. In the present study, the initial flare of androgen did not produce significant symptomatic progression.
In conclusion, a single injection of GA, a LHRH agonist, 4 weeks before TURP was found to be an effective option in controlling blood loss during TURP with minimal side-effects. This effect was supported by histological and immunohistochemical findings that it significantly reduced MVD at both prostatic suburethral and the rest of the hyperplastic prostatic tissue. However, further studies are still warranted to evaluate its effects in larger prostate volumes or in ‘risky’ patients with TURP related-bleeding comorbidities. Also, we recommended another prospective controlled study to compare the impact of GA after 1 or 3 months with finasteride on TURP related-bleeding.
Source of Funding
None.
Conflicts of interest
None declared.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- J.RassweilerD.TeberR.KuntzR.HofmannComplications of transurethral resection of the prostate (TURP)–incidence, management, and preventionEur Urol502006969980
- G.PareekM.ShevchukN.A.ArmenakasL.VasjovicD.A.HochbergJ.B.Basilloteet alThe effect of finasteride on the expression of vascular endothelial growth factor and microvessel density: a possible mechanism for decreased prostatic bleeding in treated patientsJ Urol16920032023
- G.CreaG.SanfilippoG.AnastasiC.MagnoC.VizziniA.InferreraPre-surgical finasteride therapy in patients treated endoscopically for benign prostatic hyperplasiaUrol Int7420055153
- A.V.SchallyLuteinizing hormone-releasing hormone analogs: their impact on the control of tumorigenesisPeptides20199912471262
- F.LyrdalF.O.NeidhardtDetermination of blood loss during transurethral prostatic resection. Modification of a methodScand J Urol Nephrol18198497100
- S.MarshallP.NarayanTreatment of prostatic bleeding: suppression of angiogenesis by androgen deprivationJ Urol149199315531554
- J.A.HagertyP.C.GinsbergJ.D.HarmonR.C.HarkawayPretreatment with finasteride decreases perioperative bleeding associated with transurethral resection of the prostateUrology552000684689
- S.KravchickS.CytronA.MamonovR.PeledL.LinovEffect of short-term dutasteride therapy on prostate vascularity in patients with benign prostatic hyperplasia: a pilot studyUrology73200912741278
- J.F.DonohueD.HayneU.KarnikD.R.ThomasM.C.FosterRandomized, placebo-controlled trial showing that finasteride reduces prostatic vascularity rapidly within 2 weeksBJU Int96200513191322
- A.MemisC.OzdenO.L.OzdalO.GuzelO.HanS.SeckinEffect of finasteride treatment on suburethral prostatic microvessel density in patients with hematuria related to benign prostate hyperplasiaUrol Int802008177180
- S.J.FoleyL.Z.SolomanA.W.WedderburnK.M.KashifD.SummertonV.Basketteret alA prospective study of the natural history of hematuria associated with benign prostatic hyperplasia and the effect of finasterideJ Urol1632000496498
- D.A.HochbergJ.B.BasilloteN.A.ArmenakasL.VasovicM.ShevchukG.Pareeket alDecreased suburethral prostatic microvessel density in finasteride treated prostates: a possible mechanism for reduced bleeding in benign prostatic hyperplasiaJ Urol167200217311733
- H.T.ZongX.X.PengC.C.YangY.ZhangA systematic review of the effects and mechanisms of preoperative 5alpha-reductase inhibitors on intraoperative haemorrhage during surgery for benign prostatic hyperplasiaAsian J Androl132011812818
- R.G.HahnT.FagerstromT.L.TammelaTrip O.Van VierssenH.O.BeislandA.Dugganet alBlood loss and postoperative complications associated with transurethral resection of the prostate after pretreatment with dutasterideBJU Int992007587594
- O.L.OzdalC.OzdenK.BenliS.GokkayaS.BulutA.MemisEffect of short-term finasteride therapy on peroperative bleeding in patients who were candidates for transurethral resection of the prostate (TUR-P): a randomized controlled studyProstate Cancer Prostatic Dis82005215218
- G.BartschR.S.RittmasterH.KlockerDihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasiaEur Urol372000367380
- J.KawakamiA.MoralesClinical significance of suboptimal hormonal levels in men with prostate cancer treated with LHRH agonistsCan Urol Assoc J72013E226E230
- R.J.NejatH.H.RashidE.BagiellaA.E.KatzM.C.BensonA prospective analysis of time to normalization of serum testosterone after withdrawal of androgen deprivation therapyJ Urol164200018911894
- L.SandfeldtD.M.BaileyR.G.HahnBlood loss during transurethral resection of the prostate after 3 months of treatment with finasterideUrology582001972976
- A.L.PastoreS.MarianiF.BarreseG.PalleschiA.M.ValentiniL.Paciniet alTransurethral resection of prostate and the role of pharmacological treatment with dutasteride in decreasing surgical blood lossJ Endourol2720136870
- O.LukkarinenEffect of LH-RH analogue in patients with benign prostatic hyperplasiaUrology3719919294