Abstract
Objectives: To evaluate the efficacy of solifenacin, tamsulosin oral-controlled absorption system (OCAS), and the combination of both drugs on JJ stent-related symptoms using the validated Arabic version of the ureteric stent symptom questionnaire (USSQ).
Patients and methods: In all, 260 patients who had undergone JJ stenting of the ureter for different endoscopic urological procedures were postoperatively randomly assigned into four equal groups. Patients in Group I received no treatment and served as the control group, Group II patients received tamsulosin OCAS 0.4 mg daily, Group III patients received solifenacin 5 mg daily, and Group IV patients received a combination of both drugs. Before stent removal, all patients completed the Arabic version of the USSQ.
Results: In all, 234 patients completed the study, comprised of 56 in Group I, 59 in Group II, 58 in Group III, and 61 in Group IV. Baseline characteristics and indications for JJ stenting were comparable in the four groups. There were highly significant differences in all items of the USSQ between the treatment groups and the controls, while Group II and III were comparable. The USSQ score was significantly lower in Group IV vs Groups II and III. Crossing of the distal curl of the stent to the midline had a significant positive correlation with the severity of the urinary symptoms, body pain, general health, and work performance in the medicated groups.
Conclusions: Combined therapy with tamsulosin OCAS 0.4 mg daily and solifenacin 5 mg daily is a safe and well-tolerated management for stent-related symptoms. However, stent position remains a significant factor affecting response to medical therapy and patients’ health-related quality of life.
Introduction
Endourological practice is ubiquitously associated with the use of JJ ureteric stents, which were first introduced by Zimskind et al. [Citation1] in 1967, with resultant stent-related urinary symptoms and pain that negatively influenced the patients’ general condition and quality of life (QoL) in 45–80% of patients with indwelling ureteric stents [Citation2]. The pathophysiology of these symptoms remains unclear; however, pain and LUTS caused by stent placement have been attributed to the pressure transmitted to the renal pelvis during urination, and lower ureteric and bladder spasm due to local irritation [Citation3,Citation4]. Obviously it is desirable to alleviate these associated symptoms, hence, many trials have been conducted to study the effect of pharmacological agents on stent-related symptoms, the α-blockers tolterodine and alfuzosin, and solifenacin have been investigated [Citation5Citation[6]–Citation7]. Tamsulosin acts as a selective inhibitor of α1A/1D-mediated contraction of the smooth muscles in the distal ureter, trigone, and bladder neck; relaxation of these smooth muscles decreases bladder outlet resistance and voiding pressure, with beneficial effects on stent-related LUTS [Citation8]. Solifenacin acts as a muscarinic receptor antagonist used for treatment of patients with overactive bladder (OAB) and might also be effective for stent-related symptoms [Citation7,Citation9]. Combined therapy with tamsulosin and solifenacin has been tried before and was found to significantly improve stent-related symptoms [Citation10,Citation11]. Long-term treatment with combined therapy of a fixed dose of solifenacin plus tamsulosin oral-controlled absorption system (OCAS) in men with LUTS was well tolerated and efficacious, with a low incidence of acute urine retention [Citation12]. The use of a validated tool is warranted to objectively assess the symptom complex; the ureteric stent symptoms questionnaire (USSQ) [Citation2] evaluates stent-related symptoms and their impact on QoL. The Arabic version [Citation13] is a reliable and valid tool for measuring symptoms associated with indwelling ureteric stents in our patients. There were various limitations of the previously mentioned studies, such as small sample size [Citation6], retrospective study [Citation10], and the use of non-specific scores other than USSQ [Citation11], which could be considered the standard outcome measure to evaluate the impact and compare different types of stents with better calculation of sample size with different study powers [Citation2]. Thus, the aim of the present study was to objectively evaluate the efficacy of solifenacin 5 mg daily and tamsulosin OCAS 0.4 mg daily solely and in combination in patients with JJ stents inserted following or in conjunction with different endoscopic urological procedures.
Patients and methods
This multicentre, prospective, randomised controlled study was conducted between November 2014 and June 2015 at three institutions: Benha University Hospital (Benha, Egypt), Al Adwani General Hospital (Taif, KSA) and International Medical Center (Jeddah, KSA). The study protocol was approved by the Research Ethics Committee at the Faculty of Medicine, Benha University (REC-FOMBU), which is an independently organised committee operating according to international guidelines, including the Declaration of Helsinki, Islamic Organisation for Medical Science (IOMS), the WHO, and the International Council on Harmonization and Good Practice (ICH-GCP). The inclusion criteria were patients undergoing retrograde unilateral JJ ureteric stenting before extracorporeal shockwave lithotripsy (ESWL), following ureteroscopic lithotripsy (URSL), percutaneous nephrolithotripsy (PCNL), stricture ureter, and endoscopic endopyelotomy, who agreed to be randomly allocated for treatment after obtaining an informed consent, where the procedures and possible risks were explained thoroughly. Exclusion criteria included: patients aged <18 years, pregnancy, previous application of a JJ stent, bilateral or long-term ureteric stenting, bladder pathological conditions or OAB, BPH, UTI, and those under concurrent or previous use of selective α1-blockers and/or antimuscarinic medications.
Study design
The sample size was calculated based on previous results of the USSQ validation study [Citation2] to find a difference between studied groups in one or more domain. In all, 162 patients, 38 per arm, would be sufficient to detect a difference of 20% and 30% in the mean index scores for the ‘urinary symptom’ and ‘general health’ domains, respectively, with an 80% power and a 20% attrition rate. In all, 315 patients were enrolled in the study, 132 from Benha University Hospital, 92 from Al Adwani General Hospital, and 91 from the International Medical Center. Of these patients, 260 were eligible and allocated into four equal groups, the randomisation scheme was generated in blocks (four subjects in each block) to allocate patients into four groups using the Web site Randomization.com (http://www.randomization.com) keeping the groups closely balanced and respecting the operative list in each centre. The flow of patients through the study is shown in .
Study procedure
The patients were allocated to the following groups:
| • | Group I (controls): did not take any of the study drugs. | ||||
| • | Group II: received tamsulosin OCAS 0.4 mg once daily. | ||||
| • | Group III received solifenacin 5 mg once daily. | ||||
| • | Group IV received a combination of both drugs on a daily basis. | ||||
Patients’ assessment and outcome measurement
Preoperative assessment included routine investigations for the planned procedures to be carried out under general or regional anaesthesia. A polyurethane JJ ureteric stent (Visiostar ureteric stent set, Urovision GmbH, Achenmuhl, Germany) was used in all patients, adjusting the length and calibre for each patient. A plain abdominal radiograph of the kidneys, ureters and bladder confirmed the position of the JJ stent in all patients before discharge, the mean (SD, range) duration of stent was 2.8 (1.7, 1–8) weeks. To objectively measure the primary outcome, a USSQ [Citation13] was completed on the day of stent removal to assess a number of health domains affected by stents. The questionnaire covers urinary symptoms, body pain, general health, work performance, sexual matters, and additional problems. The answers to the post-stent questionnaire would be representative of the background pre-stent condition by scoring the sum for individual questions in each section; higher USSQ scores indicate worse outcomes. Stent-related complications e.g. loop migrating into the ureter or obstruction due to encrustation, and readmissions due to complications (haematuria, symptomatic UTI, urosepsis) were also recorded.
Statistical analysis
Statistical analysis was done using SPSS software version 16.0 for windows (SPSS Inc., Chicago, IL, USA). The chi-square test was used to compare categorical data between groups. The ANOVA test was used to compare numerical data between the four groups and the post hoc Dunnett’s t-test to compare all other groups against the control group. As a measure of association between USSQ domains score and various stent factors (JJ stent length, diameter and midline crossing), Spearman’s rho nonparametric correlation was done. The Student’s t-test was used when appropriate. All statistical tests are two-sided and a P < 0.05 was considered to indicate statistical significance.
Results
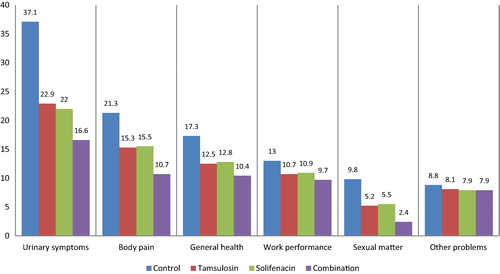
Of 260 patients, eligible and randomised to the four groups, 234 cases completed the study and were analysed, their mean (SD, range) age was 39.5 (11.6, 22–79) years. In all, 26 patients (nine from Group I, six from Group II, seven from Group III, and four from Group IV) discontinued the study (). The patients’ characteristics in the studied groups () showed that the baseline characteristics and indications for JJ stenting were similar between the studied groups. shows that there were statistically significant differences between all groups’ vs the control group for all domains of the USSQ. The difference between Group II and III was insignificant in all domains. There was a statistically significant difference between Groups II and III vs Group IV, denoting that the outcome was in favour of combined therapy, and the mean values are given in . shows that the most significant correlation was found in patients in whom the JJ stent was crossing the midline, where there was a significant positive correlation with all domains of the USSQ and total score, except for the sexual matters domain.
Table 1 The patients’ characteristics in studied groups.
Table 2 Mean (SD, range) USSQ domain scores in the four studied groups.
Table 3 Spearman’s rho correlation coefficient in 178 medicated patients with different JJ stent variables.
shows that there was a statistically significant decrease in the USSQ domain scores when the position of the distal curl of the JJ stent was not crossing the midline of the patient in the medicated groups.
Table 4 Mean (SD) USSQ domain scores associated with JJ stent distal curl position in relation to midline among the medicated groups.
For the presence of side-effects in the medicated patients, there were 13 cases of dry mouth [six of 58 patients (10%) in the solifenacin group and seven of 61 (11%) in the combined therapy group], with a statistically significant difference between groups (P = 0.004). Retrograde ejaculation was reported in five patients [two of 33 patients (6%) in the tamsulosin group and three of 37 (8%) in the combined therapy group], with a statistically insignificant difference between groups (P = 0.158).
In the present study, for the stent-related complication items (covered in the questionnaire and scored), all patients in the control group reported at least one attack of mild haematuria (rare attacks in 12, sometimes in 37, most of the times in six, and always present in one). In the tamsulosin group, 17/59 patients (29%) had haematuria, where it was rare in 14 and sometimes in three. Rare attacks were found in four of 58 (7%) and four of 61 (7%) patients in both the solifenacin and combined therapy groups, respectively, with a statistically significant difference between groups (P < 0.001). Symptomatic UTI was found in eight patients (14%) in the control group, four (7%) in the tamsulosin group, three (5%) in the solifenacin group, and six (10%) in the combined therapy group, with a statistically nonsignificant difference between the groups (P = 0.339). The need to consult the treating doctor for a prescription of another course of antibiotics was needed in eight patients (14%) in the control group, five (8%) in the tamsulosin group, three (5%) in the solifenacin group, and four (7%) in the combined therapy group, with a statistically significant difference between groups (P = 0.036). Readmission due to the presence of stent-related complications was necessary in four patients (7%) in the control group, while none of the medicated patients required readmission, with a statistically significant difference between groups (P < 0.001). The score for additional problems, such as development of fever with the need for another course of antibiotics or the need for consultation and readmission because of complications were not significantly different between the medicated groups ().
Discussion
Ureteric stents are considered an integral part of endoscopic procedures, but the resultant symptoms of discomfort and pain impair QoL, and many patients report that this to be the worst part of the procedure. Bothersome symptoms and pain related to stents are reported in 80% of patients, with sexual dysfunction and reduced work performance reported in 32% and 58% of those patients, respectively [Citation2]. The commonly reported symptoms include flank pain, haematuria, dysuria, frequency, and urgency [Citation14]. The pain and LUTS caused by stent placement have been attributed to local irritation of the bladder and ureteric mucosa causing smooth muscle spasm and pain due to high pressure ureteric reflux [Citation3,Citation4]. A unified instrument merging these symptoms was developed [Citation2] to assess stent symptoms; the USSQ evaluates six domains of urinary symptoms, body pain, general health, work performance, sexual matters and additional problems, totalling the summary scores from each section, with higher scores corresponding to worse outcomes. The Arabic version of the USSQ [Citation13] was previously validated and considered a reliable tool for evaluating symptoms and health-related QoL in patients with ureteric stents, hence, was used in the present study. It allows for meaningful comparison of various interventions in the ongoing effort to improve stent tolerance. Studies using monotherapy with selective α1 blockers (e.g. tamsulosin) have reported improved urinary symptoms, flank pain, pain during voiding, visual analogue pain scale (VAPS), and QoL [Citation8,Citation15]. In addition, antimuscarinics (e.g. solifenacin) have been tried to alleviate these symptoms [Citation7]. In a recent randomised controlled trial (RCT) including 149 patients [Citation16] comparing an α-blocker (tamsulosin) and anticholinergic (solifenacin) as monotherapy for treatment of ureteric stent-related symptoms compared with placebo, the authors found that the total USSQ score was 61 in the solifenacin group, 76 in the tamsulosin group, and 83 in the control group (P < 0.001); with superiority of the solifenacin over the tamsulosin group (P < 0.05).
The efficacy of combined therapy of both drugs in different doses have been assessed in multiple studies [Citation10,Citation11,Citation17]. Lim et al. [Citation10] assessed the effectiveness of a selective α1-blocker (tamsulosin 0.2 mg) and antimuscarinic (solifenacin 5 mg) in improving LUTS in patients with ureteric stents using the IPSS, IPSS/QoL and VAPS questionnaires. In another study [Citation11], tamsulosin OCAS 0.4 mg daily, solifenacin 10 mg daily, and a combination of both medications were tried in a RCT using IPSS/QoL, overactive bladder questionnaire (OAB-q), and VAPS questionnaire. The previous two studies concluded that combined therapy with tamsulosin and solifenacin improved both irritative and obstructive symptoms and should be strongly considered for patients who complain of stent-related symptoms. α-Blockers and antimuscarinics have been shown to have a synergistic effect and be more effective than either medication alone in reducing stent-related symptoms [Citation17,Citation18].
In the present study, the efficacy of tamsulosin OCAS 0.4 mg daily, solifenacin 5 mg daily, and a combination of both medications were assessed using the USSQ. In addition, we analysed different stent factors e.g. length, diameter, and position, and correlated these factors with patient symptoms. Patients with different urological procedures were included in the present study. However, the baseline patients’ characteristics, indications for stent insertion, and different stent factors (diameter, length, and position) were similar in the four groups. Reducing the dose of solifenacin from 10 mg as in the previously mentioned study [Citation11] to 5 mg decreases the potential side-effects of these medications, especially with elderly patients, who may have coexisting BOO, as these may worsen their symptoms and exacerbate BOO [Citation19,Citation20].
The results of the present study show that the medicated groups had significant improvements in the USSQ compared with the control group. There was an insignificant difference between tamsulosin and solifenacin for the USSQ domains, while combined therapy significantly improved stent-related symptoms compared with monotherapy with either one of the drugs. These results are in agreement with the previously mentioned studies [Citation10,Citation11]. Our medicated groups received the same dose as a previously mentioned RCT [Citation16] with some differences, as they included only patients aged 20–50 years, which may explain our higher total USSQ score, and no combined therapy group. Moreover, the authors found some superiority for solifenacin over tamsulosin monotherapy, which was not the case in the present study. In the medicated groups in the present study there were no serious adverse events, although dry mouth was reported in 13 patients [six in the solifenacin group (10%) and seven in the combined therapy group (11%)]. Retrograde ejaculation was found in five patients, two men in the tamsulosin group (6%) and three in the combined therapy group (8%). There were no cases of acute urinary retention. These side-effects reported in our present patients were comparable with those reported in the SATURN study [Citation20], and were consistent with the known safety profiles of each individual drug [Citation21].
Analysing different stent factors, including the use of different stent sizes, lengths, and position of the distal end of the stent even in the combined therapy group, showed that the position of the stent with respect to the midline remains the most significant factor affecting USSQ scores, although the combined therapy group had significantly decreased stent-related symptoms. This shows that correct stent placement is essential to minimise stent-related symptoms and the role of medication here is to ameliorate stent morbidities, which affect all domains of the USSQ. The result of the present study are in agreement with the study of Lee et al. [Citation22], who in a prospective randomised study of 53 cases, compared combined therapy with tamsulosin 0.2 mg once daily and tolterodine 4 mg daily with a placebo group. The authors concluded that the correct placement of the stent was more important than medication for lessening stent-related storage symptoms. The length and position of the stent have to be adequate, as recommended by many authors and patients, with a crossing stent there will be at a higher risk of post-procedural morbidity requiring early management [Citation23Citation[24]–Citation25]. In addition, some authors recommend cautious use and the stent dwell-time should be minimised [Citation26].
Limitations of the present study were the lack of homogeneity of patients due to including different procedures with the need for stents for variable lengths of time, but the different procedures in the study were distributed similarly between the groups (). In addition, elderly patients were included that could have some impact on the total USSQ score. Further studies on a larger scale are needed to verify which cohorts of patients require medication in the presence of a ureteric stent or if the medication may be used at the discretion of the provider.
Conclusion
The use of combined therapy with tamsulosin OCAS 0.4 mg daily and solifenacin 5 mg daily is a safe and well tolerated for stent-related symptoms. However, stent position with respect to the midline remains a significant factor affecting response to medical therapy and patients’ QoL.
Conflict of interest
No conflict of interest.
Source of Funding
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- P.D.ZimskindT.R.FetterJ.L.WilkersonClinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopicallyJ Urol971967840844
- H.B.JoshiN.NewnsA.StainthorpeR.P.MacDonaghF.X.KeeleyJr.A.G.TimoneyUreteral stent symptom questionnaire: development and validation of a multidimensional quality of life measureJ Urol169200310601064
- W.M.SamehA.A.EidPressure transmission through ureteric stents: a novel in vivo human studyUrology792012766770
- J.H.SiggersS.WatersJ.WattisL.CummingsFlow dynamics in a stented ureterMath Med Biol262009124
- R.YakoubiM.LemdaniM.MongaA.VillersP.KoenigIs there a role for alpha-blockers in ureteral stent related symptoms? A systematic review and meta-analysisJ Urol1862011928934
- S.C.ParkS.W.JungJ.W.LeeJ.S.RimThe effects of tolterodine extended release and alfuzosin for the treatment of double-j stent related symptomsJ Endourol23200919131917
- Y.J.LeeK.H.HuangH.J.YangH.C.ChangJ.ChinT.K.YangSolifenacin improves double-J stent-related symptoms in both genders following uncomplicated ureteroscopic lithotripsyUrolithiasis412013247252
- C.J.WangS.W.HuangC.H.ChangEffects of specific α-1A/1D blocker on lower urinary tract symptoms due to double-J stent: a prospectively randomized studyUrol Res372009147152
- M.D.VardyH.D.MitchesonT.A.SamuelsJ.D.WegenkeS.Forero-SchwanhaeuserT.S.Marshallet alEffects of solifenacin on overactive bladder symptoms, symptom bother and other patient-reported outcomes: results from VIBRANT – a double-blind, placebo-controlled trialInt J Clin Pract63200917021714
- K.T.LimY.T.KimT.Y.LeeS.Y.ParkEffects of tamsulosin, solifenacin, and combination therapy for the treatment of ureteral stent related discomfortsKorean J Urol522011485488
- E.ShalabyA.F.AhmedA.MaaroufI.YahiaM.AliA.GhobishRandomized controlled trial to compare the safety and efficacy of tamsulosin, solifenacin, and combination of both in treatment of double-J stent-related lower urinary symptomsAdv Urol20132013 752382
- M.J.DrakeC.ChappleR.SokolM.OelkeK.TraudtnerM.Klaveret alLong-term safety and efficacy of single-tablet combinations of solifenacin and tamsulosin oral controlled absorption system in men with storage and voiding lower urinary tract symptoms: results from the NEPTUNE Study and NEPTUNE II open-label extensionEur Urol672015262270
- A.R.El-NahasM.M.ElsaadanyM.TharwatA.MosbahA.MetwallyF.X.KeeleyJr.et alValidation of the Arabic linguistic version of the ureteral stent symptoms questionnaireArab J Urol122014290293
- R.DamianoA.OlivaC.EspositoM.De SioR.AutorinoM.D’ArmientoEarly and late complications of double pigtail ureteral stentUrol Int692002136140
- R.DamianoR.AutorinoM.De SioA.GiacobbeI.M.PalumboM.D’ArmientoEffect of tamsulosin in preventing ureteral stent-related morbidity: a prospective studyJ Endourol222008651656
- A.R.El-NahasM.TharwatM.ElsaadanyA.MosbahM.A.GaballahA randomized controlled trial comparing alpha-blocker (tamsulosin) and anticholinergic (solifenacin) in treatment of ureteral stent-related symptomsWorld J Urol201510.1007/s00345-015-1704-3 [Epub ahead of print]
- L.ZhouX.CaiH.LiK.J.WangEffects of α-blockers, antimuscarinics, or combination therapy in relieving ureteral stent-related symptoms: a meta-analysisJ Endourol292015650656
- B.H.ChewC.SeitzImpact of ureteral stenting in ureteroscopyCurr Opin Urol2620167680
- C.ChappleV.KhullarZ.GabrielJ.A.DooleyThe effects of antimuscarinic treatments in overactive bladder: a systematic review and meta-analysisEur Urol482005526
- P.Van KerrebroeckF.HaabJ.C.AnguloV.VikF.KatonaA.Garcia-Hernandezet alEfficacy and safety of solifenacin plus tamsulosin OCAS in men with voiding and storage lower urinary tract symptoms: results from a phase 2, dose-finding study (SATURN)Eur Urol642013398407
- M.C.LiZ.Y.WangJ.YangX.L.GuoT.WangS.G.Wanget alEfficacy and safety of solifenacin plus tamsulosin oral controlled absorption system in men with lower urinary tract symptoms: a meta-analysisAsian J Androl172015124134
- S.J.LeeC.YooC.Y.OhY.S.LeeS.T.ChoS.H.Leeet alStent position is more important than alpha-blockers or anticholinergics for stent-related lower urinary tract symptoms after ureteroscopic ureterolithotomy: a prospective randomized studyKorean J Urol512010636641
- A.M.Al-KandariT.F.Al-ShaijiH.ShaabanH.M.IbrahimY.H.ElshebinyA.A.ShokeirEffects of proximal and distal ends of double-J ureteral stent position on postprocedural symptoms and quality of life: a randomized clinical trialJ Endourol212007698702
- G.GiannariniF.X.KeeleyJr.F.ValentF.ManasseroA.MogorovichR.Autorinoet alPredictors of morbidity in patients with indwelling ureteric stents: results of a prospective study using the validated Ureteric Stent Symptoms QuestionnaireBJU Int1072011648654
- M.S.Al-MarhoonO.ShareefP.KrishnaK.P.VenkiteswaranComplications and outcomes of JJ stenting of the ureter in urological practice: a single-centre experienceArab J Urol102012372377
- R.C.CalvertK.Y.WongS.V.ChitaleS.O.IrvingM.NagarajanC.S.Biyaniet alMulti-length or 24 cm ureteric stent? A multicentre randomized comparison of stent-related symptoms using a validated questionnaireBJU Int111201310991104