Abstract
Objective To explore the evaluation, treatment and impact of hyperprolactinaemia on male infertility and testicular function, as hyperprolactinaemia is commonly detected during the evaluation of infertile men.
Methods A literature search was performed using MEDLINE/PubMed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify all studies exploring hyperprolactinaemia in male infertility.
Results Elevated levels of serum prolactin have a detrimental effect on male reproduction through inhibition of the pulsatile release of gonadotrophins from the anterior pituitary gland, and a direct effect on spermatogenesis. Treatment of confirmed hyperprolactinaemia with dopamine agonists leads to significant improvements in both semen parameters and hormone levels.
Conclusion Hyperprolactinaemia, both directly and indirectly, has a negative effect on sperm production, and its detection and management in men seeking fertility is mandatory.
Introduction
Infertility is classically defined as the inability to conceive after at least 1 year of regular unprotected intercourse. It is a common medical condition affecting between 9% and 25% of couples worldwide [Citation1,Citation2]. A male factor contributes to roughly half the cases of infertility amongst couples [Citation3]. Establishing a precise diagnosis via a thorough history, examination and investigative protocol is essential for optimal male infertility management. During evaluation, the specialist investigates for the presence of endocrine dysfunction that may contribute to a patient’s infertility. Current guidelines indicate hormone evaluation of infertile men in the presence of abnormal semen analysis, symptoms of hypogonadism, or other clinical findings suggestive of a specific endocrinopathy, such as gynaecomastia or testicular atrophy [Citation4].
Hyperprolactinaemia is amongst the endocrine disorders known to influence male infertility. It is a common medical condition present in ∼1% of the general population worldwide [Citation5]. Hyperprolactinaemia in men is defined by the presence of a high serum prolactin level of >15 µg/L. It can result from physiological or pathological conditions. Stress and exercise can cause small increases in prolactin levels and are important causes of physiological hyperprolactinaemia [Citation6]. Medication-induced hyperprolactinaemia is usually associated with prolactin levels ranging from 25 to 100 µg/L, but metoclopramide, risperidone, and phenothiazines can lead to prolactin levels of >200 µg/L [Citation6].
Prolactinomas (lactotroph adenomas) are the most common pathological cause of hyperprolactinaemia and account for ∼40% of pituitary adenomas [Citation7]. The diagnosis is more commonly made in women than men due to the effect of hyperprolactinaemia on the female menstrual cycle giving an earlier indication of hormonal imbalance. Prolactinomas can be microadenomas (<1 cm in diameter) or macroadenomas (>1 cm in diameter), and the level of serum prolactin measured is directly proportional to the size of the adenoma [Citation7].
Whilst hyperprolactinaemia is prevalent in up to 11% of infertile males [Citation8], it is a diagnosis that is often missed because of its subtle clinical manifestations. In the present systematic review, we examine the available evidence for the effect of hyperprolactinaemia on male infertility, and highlight the approach for the evaluation and treatment of hyperprolactinaemia using clinical case-based scenarios.
Methods
Search strategy
This study was designed according to modified guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation9]. PubMed/MEDLINE databases were searched using the following search terms in titles and abstracts: ‘hyperprolactinemia’, ‘male infertility’, ‘dopamine agonists’, ‘prolactin’, ‘medical treatment for male infertility’, ‘endocrine causes of male infertility’. The search was limited to studies performed on humans. The literature was searched from inception to 18 August 2017.
Study selection
The generated list of articles was screened by title and abstract by the authors (Z.D. and S.A.) and then relevant full papers were examined. Review articles were also explored to find additional appropriate papers. The exclusion criteria were based on gender (females), species (other animals), and study methods (retrospective, case report, editorial or commentary). Data were then extracted, cross-checked, and verified.
Data extraction
Eligible studies were reviewed and the following data were abstracted: (i) first author’s name, (ii) year of publication, (iii) country where the study was performed, (iv) study design, (v) number of participants in the study and control groups, (vi) type of medication, (vii) duration of treatment, (viii) baseline and follow-up semen parameters/male fertility status.
Results
Overall, 39 articles were found following the multi-database search. After screening of titles and abstracts, 17 articles were assessed in full. Of these, nine articles were excluded because they did not meet the inclusion criteria. Therefore, eight articles were found to be eligible for inclusion in the systematic review ().
Study characteristics
The included studies were published between 1981 [Citation10] and 2011 [Citation11]. The range of treatment duration was from 2.25 [Citation12] to 24 months [Citation13]. Study designs of included trials were comparative studies [Citation13Citation[14]–Citation15] and prospective observational studies [Citation10Citation[11]–Citation12,Citation16,Citation17]. Selected studies enrolled patients with hyperprolactinaemia and oligospermia [Citation12] or hyperprolactinaemia and infertility [Citation17]. Medications assessed for the treatment of hyperprolactinaemia where bromocriptine [Citation10,Citation12,Citation15,Citation17], cabergoline [Citation11,Citation13Citation[14]–Citation15] and quinagolide [Citation16]. Characteristics of the included clinical trials are shown in [Citation10Citation[11]Citation[12]Citation[13]Citation[14]Citation[15]Citation[16]–Citation17].
Table 1 Studies assessing the effect of hyperprolactinaemia treatment on male fertility.
Hypothalamic–pituitary–gonadal axis (HPG)
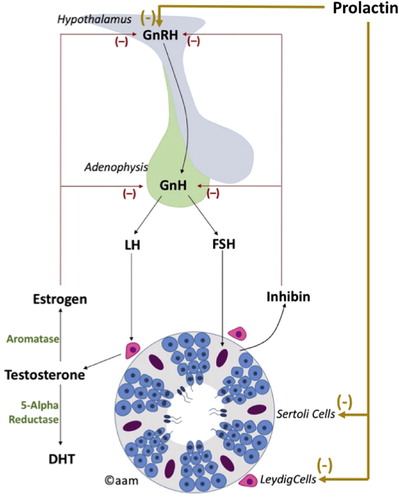
An understanding of the HPG axis is important for understanding the pathophysiology of pituitary dysfunction in male infertility. Testicular function is regulated by the hypothalamus and pituitary gland (). The hypothalamus secretes GnRH, which reaches the anterior pituitary via the hypothalamo-hypophyseal portal circulation to stimulate production of the glycoprotein hormones LH and FSH. FSH and LH are consequently secreted into the circulation for their stimulatory actions on the testes. FSH acts on Sertoli cells triggering spermatogenesis and hormone synthesis, primarily inhibin. LH binds to the LH receptors on Leydig cells stimulating steroidogenesis and testosterone production. There is evidence suggesting that FSH may stimulate testosterone production by Leydig cells secondary to the release of activating hormones from Sertoli cells [Citation18].
The secretion of GnRH by the hypothalamus is pulsatile in nature triggering a response in LH and consequently testosterone synthesis [Citation19]. This circadian rhythm of hormone release is essential for human health and well-being.
Testosterone is subsequently aromatised to oestradiol that exerts a negative feedback on the hypothalamus and the pituitary gland, resulting in decreased production of GnRH, FSH and LH, consequently maintaining testosterone in its optimal range. Inhibin also exerts negative feedback on the pituitary gland decreasing LH and FSH production [Citation18].
Pathophysiological influence of prolactin on male reproduction
Prolactin inhibits pulsatile GnRH secretion and consequently inhibits the pulsatile release of FSH, LH and testosterone [Citation19]. This results in marked effects on spermatogenesis ranging from alteration in sperm quality to complete spermatogenic arrest [Citation19]. As a result, the patient may present with secondary hypogonadism or male infertility. Furthermore, prolactin may also impact male fertility through a direct effect on spermatogenesis. Studies have identified prolactin receptors on Leydig cells, Sertoli cells and epithelial cells of efferent ducts, suggesting a potential role for prolactin in promoting steroidogenesis, spermatogenesis and secretory/adsorptive functions of male reproductive organs [Citation20Citation[21]–Citation22].
Clinical and investigative evaluation
Clinical case scenario 1
A 32-year-old man presents complaining of infertility of >12 months duration and confirms he is having regular intercourse with this wife. He denies any past medical or surgical history of relevance. He is not taking any prescribed or over the counter medications. Clinical examination was unremarkable. Laboratory investigation revealed that he had oligospermia. His hormone evaluation revealed an FSH level of 2.1 IU/L, LH level of 3.2 IU/L, testosterone level of 13.5 mmol/L, and prolactin level of 34 µg/L.
Clinical case scenario 2
A 32-year-old man presents complaining of infertility of >12 months duration. He admitted that he recently started complaining of decreased libido and erectile dysfunction over the preceding 6 months. He is not taking any prescribed or over the counter medications. Upon evaluation, he was found to have severe oligoasthenozoospermia. His hormone evaluation revealed an FSH level of 1.3 IU/L, LH level of 1.1 IU/L, testosterone level of 6.7 mmol/L, and prolactin level of 986 µg/L.
The rationale behind presenting these two commonly encountered clinical scenarios is to highlight the precise indications for further evaluation of hyperprolactinaemia. In the first scenario, the mild elevations in prolactin level may be attributed to physiological causes such as stress, exercise or excessive venepuncture. It might also be secondary to concurrent intake of prescription medications such as antipsychotic agents, metoclopramide and cimetidine, but it is also important to ask about additional over the counter supplements and any herbal preparations. In this case the prolactin level measurement should be repeated on three occasions after the assumed cause has been dealt with; if any one of those three measurements is normal then it can be assumed that this is stress induced hyperprolactinaemia or that the cause (such as over the counter medication) has been addressed. Alternatively a 5-point cannulated prolactin profile from 0800 h to 1200 h has also be advocated [Citation23], when the prolactin may then fall to normal, although this necessitates a prolonged outpatient or day case admission.
In the second scenario, the patient presents with symptoms of hypogonadism together with more than a twofold increase in the prolactin level. This probably indicates a true pathological cause of hyperprolactinaemia, such as a prolactinoma. Therefore, a more aggressive approach to evaluation should be undertaken. It should be noted that low secreting microprolactinomas can cause mild elevation of prolactin levels and some medications can cause high levels of prolactin exceeding 200 µg/L.
In both scenarios, the clinician should pay attention to two pitfalls in serum prolactin measurement. Firstly, the ‘hook’ effect that occurs when a very high serum prolactin level (>5000 µg/L) saturates both the capture and signal antibodies used in measurement assays preventing the binding of the two in a sandwich [Citation24]. The result is an apparent modest elevation in prolactin level. This can be avoided by repeating the assay using a 1/100 dilution of serum.
Secondly, macroprolactinaemia is hyperprolactinaemia due to excess macroprolactin with normal concentrations of monomeric prolactin [Citation25]. Macroprolactin is a non-bioactive prolactin isoform usually composed of a prolactin monomer and an IgG molecule having a prolonged clearance rate similar to that of immunoglobulins. This isoform is clinically non-functional and therefore not of clinical significance, but it interferes with immunological assays used for the detection of prolactin that may give a falsely elevated prolactin result [Citation26]. Therefore, macroprolactinaemia may lead to misdiagnosis and mismanagement of hyperprolactinaemic patients if not recognised. Current best practice recommends that all sera with elevated total prolactin concentrations are sub-fractionated using polyethylene glycol precipitation to provide an accurate measurement of the bioactive monomeric prolactin content [Citation27].
Additional medical causes of hyperprolactinaemia include renal failure, hypothyroidism, and parasellar tumours in patients with symptomatic hyperprolactinaemia. Patients with renal insufficiency may have a moderate increase in prolactin levels caused by impaired renal degradation of prolactin and altered central prolactin regulation [Citation28]. Some patients with primary hypothyroidism also have a moderate increase in prolactin levels [Citation29]. Primary hypothyroidism can cause pituitary hyperplasia that may mimic a pituitary tumour that is reversible by treatment with levothyroxine.
Prolactin secretion is inhibited by hypothalamic dopamine (DA); hence compression of the pituitary stalk by a non-prolactin-secreting pituitary tumour or other parasellar mass will lead to hyperprolactinaemia.
In the absence of physiological, pharmacological or other secondary causes of hyperprolactinaemia, imaging (preferably MRI) of the pituitary fossa is recommended to establish whether a prolactin-secreting pituitary tumour or other lesion is present [Citation27]. Whereas serum prolactin levels between 20 and 200 μg/L can be found in patients with hyperprolactinaemia due to any cause, prolactin levels >200 μg/L usually indicate the presence of a prolactinoma [Citation27].
Management
Treatment of hyperprolactinaemia depends on its cause and whether the patient is symptomatic.
Medication induced hyperprolactinaemia
The second most frequent cause of hyperprolactinaemia is medication, with neuroleptics/antipsychotic agents being the drugs most commonly involved [Citation30]. Usually prolactin levels increase slowly after the introduction of these agents, and it takes 3 days for levels to return to normal after drug discontinuation [Citation27]. Most of men with drug-induced hyperprolactinaemia remain asymptomatic; however, some men may present with low libido and erectile dysfunction.
Medication-induced hyperprolactinaemia is usually associated with low or modest prolactin levels (25–100 μg/L), but metoclopramide, risperidone, and phenothiazines can lead to prolactin levels of >200 μg/L [Citation31].
Verapamil, opiates and cocaine can cause mild elevations in prolactin levels that usually does not warrant treatment [Citation31]. It should be emphasised that it is important to ask the patient about herbal, homeopathic and over-the-counter medication that may contribute or cause the increased prolactin level.
According to Endocrine Society guidelines, the first step in treatment of medication-induced hyperprolactinaemia is to stop the drug if this is clinically feasible [Citation27]. If this is not possible, a drug with a similar action that does not cause hyperprolactinaemia should be substituted, and if this is not feasible then consider the cautious administration of a DA agonist in consultation with the patient’s physician [Citation27].
Hypothalamic and pituitary disease
Compression of the pituitary stalk by a tumour or infiltrative disease of the hypothalamus or pituitary gland can lead to hyperprolactinaemia [Citation32]. If removal of the non-functional adenoma or mass is not possible, the hyperprolactinaemia should be treated with a DA agonist, although non-functional tumour shrinkage is rare.
Hypothyroidism
Treatment of hypothyroidism and restoration of normal thyroid-stimulating hormone will lead to normalisation of prolactin levels and does not warrant any additional treatment [Citation27].
Macroprolactinaemia
Macroprolactinaemia has no clinical significance; it is a benign condition that does not require treatment.
Prolactinomas
Studies of the natural history of microadenomas (<1 cm) show that 95% do not enlarge during 4–6 years of observation [Citation33]. Treatment may not be warranted unless the patient is symptomatic (hypogonadism), although in real terms most patients are treated.
Macroadenomas (>1 cm) more commonly present in men after the onset of neurological symptoms such as visual impairment or headache. In this scenario, DA agonist treatment needs to be implemented urgently, particularly if visual field loss is apparent. Similarly for prolactinomas extending outside of the sella, as these lesions are likely to continue to grow and eventually cause neurological or visual symptoms.
The Endocrine Society recommends DA agonist therapy as first-line treatment of micro- and macroprolactinomas [Citation27]. In a systematic review conducted by the society, DA agonists lowered prolactin levels, decreased tumour size, and restored gonadal function in patients with symptomatic prolactinomas [Citation34].
Cabergoline is the preferred DA agonist because it has a higher efficacy in normalising prolactin levels, a higher frequency of pituitary tumour shrinkage, and fewer side-effects [Citation34]. However, for large tumours a short acting DA analogue, such as bromocriptine or quinagolide, may be preferential for the initial therapy in case of a resultant cerebrospinal fluid leak that can be rapidly reversed by stopping the medication. A cerebrospinal fluid leak is less readily reversed by stopping cabergoline, which has such a long half-life.
In a placebo-controlled study, treatment with cabergoline at a dose of 0.125–1.0 mg twice weekly for 12–24 months normalised prolactin levels in 95% of patients with prolactin-secreting microadenomas [Citation35]. Another prospective study investigating 26 treatment-naive patients with macroprolactinomas who received treatment with cabergoline 0.25–2 mg weekly for 6 months revealed successful normalisation of prolactin levels in 81% of patients, with 92% displaying significant tumour shrinkage [Citation36].
Normalisation of prolactin levels was also achieved in 80% of patients with macro- or microadenomas after treatment with bromocriptine, cabergoline, or other DA agonists [Citation37].
Medications
Cabergoline
Cabergoline is an ergot DA agonist given one or twice a week. The initial dose is 0.5 mg once weekly or 0.25 mg twice weekly. The weekly dose may be increased by 0.5 mg/week at 4-weekly intervals until an optimal therapeutic response is achieved. The most common side-effects are nausea, postural hypotension, and mental fogginess. Cabergoline might cause valvular heart disease when given in very high doses in the treatment of Parkinson disease and it has been suggested that echocardiography should be undertaken; however, the low dose given in hyperprolactinaemia is not associated with an excess cardiac risk [Citation38].
Bromocriptine
Bromocriptine is a semi-synthetic ergot alkaloid derivative. The initial dose is 1.25–2.5 mg daily, which may be increased by 2.5 mg daily as tolerated, every 2–7 days until an optimal response is achieved (range 2.5–15 mg/day). Most common side-effects are dizziness, headache, and nausea [Citation39].
Quinagolide
Quinagolide is a non-ergot derived DA agonist that has DA D2 receptor selectivity. As a specific inhibitor of prolactin secretion, quinagolide offers a favourable tolerability profile and a prolonged duration of action. An initial dose is titrated from 0.025 to 0.075 mg/day over a 7-day period. The most common side-effects include: loss of appetite, nausea, and headache [Citation40].
Prolactinoma follow-up
Patients should be followed regularly to assess medication tolerability and side-effects, and to assess the prolactin level as a measure of treatment response.
A decrease in the prolactin level would lead to continuation of the DA agonist at the same dose; however, if the level does not decrease then the DA agonist should be increased [Citation27].
For macroadenomas, pituitary MRI should be repeated after 6 months to evaluate tumour shrinkage. After 1 year of treatment, and if the prolactin level is within the normal laboratory reference range and there is prolactinoma shrinkage, then the DA agonist dose may be decreased gradually particularly in those patients with troubling side-effects [Citation27].
For microprolactinomas that can no longer be visualised on MRI and the prolactin levels are normal, the DA agonist can be discontinued and the patient followed-up with prolactin measurements [Citation27,Citation41]; however, therapy is lifelong for those with macroprolactinomas.
Men with hyperprolactinaemia and hypogonadism who cannot tolerate or who do not respond to DA agonists may consider either surgery or radiotherapy.
Surgery and radiation therapy
Trans-sphenoidal surgery is recommended for symptomatic patients with prolactinomas who cannot tolerate high doses of DA agonists or who are not responsive to medical therapy. Side-effects of surgery include: hypopituitarism, infection, diabetes insipidus, and cerebrospinal fluid leak.
Radiotherapy should be reserved for resistant prolactinomas. Prolactin levels normalise in about one-third of patients treated with radiation [Citation42]. Radiation therapy side-effects include: hypopituitarism and, rarely, cranial nerve damage or second tumour formation [Citation43].
Effect of hyperprolactinaemia and its treatment on male infertility
Few studies have investigated the impact of hyperprolactinaemia on male reproduction and sexual function [Citation44Citation[45]Citation[46]Citation[47]Citation[48]Citation[49]Citation[50]–Citation51]. Despite the known detrimental influence of hyperprolactinaemia on the HPG axis, there are few studies investigating the effect hyperprolactinaemia on semen quality and those reported are contradictory. Gonzales et al. [Citation47] assessed serum prolactin levels in 60 men attending an infertility service. The authors reported significantly higher serum prolactin levels in patients with azoospermia [mean (SD) 44.2 (4.7) µg/L] than those with normozoospermia [mean (SD) 15.9 (1.6) µg/L; P < .001]. In another study, of similar design, serum prolactin levels were inversely correlated with sperm concentration. The mean (SEM) prolactin concentration amongst azoospermic and oligospermic men was 59.9 (7.3) µg/L and 54.7 (8.5) µg/L, respectively, which were significantly higher than normozoospermic men at 34.2 (1.9) µg/L (P < .001) [Citation46]. Studies investigating testicular histopathology results in infertile men with hyperprolactinaemia reported varying degrees of impairment of spermatogenesis ranging from hypospermatogenesis to complete absence of germ cells [Citation44,Citation45]. Lotti et al. [Citation49] addressed ejaculatory and erectile function in men with hyperprolactinaemia revealing a negative association between prolactin and delayed ejaculation using the premature ejaculation diagnostic tool. There was no significant correlation between serum prolactin levels and erectile function. Detailed studies on semen quality including sperm motility appear lacking. Conversely others have failed to find hyperprolactinaemia effects on semen parameters. Okada et al. [Citation50] investigated the effect of hyperprolactinaemia on sperm function in 264 men with oligoasthenoteratozoospermia. The authors reported no significant correlations between abnormal semen values and hyperprolactinaemia. Soler Fernández et al. [Citation48] measured serum prolactin levels in 34 patients with normospermia, 69 with oligospermia, 26 with azoospermia, and 18 with purely asthenozoospermia. Despite finding a 6.19% lower incidence of hyperprolactinaemia in the normospermia group, there were no statistically significant differences in serum prolactin levels between the different patient groups. These contradictory results could be attributed to the fact that male infertility is often multifactorial and hence studies with non-uniform methodologies may have been affected by confounding factors.
Studies investigating the effects of treating hyperprolactinaemia on male fertility are shown in . Treatment with cabergoline, bromocriptine and quinagolide normalised prolactin levels in all the studies shown in , regardless of the underlying cause of hyperprolactinaemia. The effect of treatment on hypogonadism and semen analysis parameters was evident in all but one of those studies [Citation17]. De Rosa et al. [Citation15] compared cabergoline and bromocriptine for the treatment of hyperprolactinaemia. In that study 17 men with macroprolactinoma were treated with cabergoline (seven men) or bromocriptine (10 men). After 6 months of treatment, a significant improvement in all semen analysis parameters was recorded in patients treated with either cabergoline or bromocriptine. However, the improvements in seminal fluid parameters were more evident and rapid in patients treated with cabergoline. There was a significant increase in serum testosterone, from a mean (SEM) of 3.7 (0.3) to 5.3 (0.2) µg/L. Side-effects were more common in patients taking bromocriptine. Nishimura et al. [Citation17] reported the only study that did not show any improvement in semen analysis after treatment with bromocriptine. The men in that study had infertility and a mildly elevated prolactin level just above the upper limit of the reference range, and no patient had pituitary disease. In the study, bromocriptine normalised the prolactin level but did not have an effect on semen analysis. This might be explained by the fact that the mildly elevated prolactin level, the causes of which were not determined, was not the immediate cause of the infertility.
Improvements in sexual function were more evident and rapid in patients treated with cabergoline than bromocriptine [Citation15].
Conclusions
Hormone assessment is an integral part of male infertility evaluation. Amongst the different components of the HPG axis, prolactin plays an important role in the pathophysiology of the male infertility potentially altering testicular function and semen production. Hyperprolactinaemia has been linked to a state of hypogonadism and a reduction in semen quality. Treatment of hyperprolactinaemia leads to an improvement in reproductive function, health and well-being. Cabergoline is the DA agonist of choice, with an improvement in semen quality and sexual function, and has a low side-effect profile. The precise effect and mechanisms of action of prolactin on semen quality and quantity are not fully understood and need further clarification.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Financial disclosure
This work was unfunded.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- M.N.MascarenhasS.R.FlaxmanT.BoermaS.VanderpoelC.D.MathersG.A.StevensTrends in primary and secondary infertility prevalence since 1990: a systematic analysis of demographic and reproductive health surveysLancet3812013S90
- J.BoivinL.BuntingJ.A.CollinsK.G.NygrenInternational estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical careHum Reprod22200715061512
- V.M.BrughL.I.LipshultzMale factor infertility: evaluation and managementMed Clin North Am882004367385
- American Urological Association. Optimal Evaluation of the Infertile Male: best practice statement reviewed and validity confirmed 2011. Available at: http://www.auanet.org/guidelines/male-infertility-optimal-evaluation-(reviewed-and-validity-confirmed-2011). Accessed October 2017.
- Biller BM, Luciano A, Crosignani PG, Molitch M, Olive D, Rebar R et al. Guidelines for the diagnosis and treatment of hyperprolactinemia. J Reprod Med 1999; 44 (Suppl.):1075–84.
- A.MajumdarN.S.MangalHyperprolactinemiaJ Hum Reprod Sci62013168175
- L.KatznelsonA.KlibanskiProlactinomasCancer Treat Res8919974155
- P.SinghM.SinghG.CugatiA.K.SinghHyperprolactinemia: an often missed cause of male infertilityJ Hum Reprod Sci42011102103
- D.MoherA.LiberatiJ.TetzlaffD.G.AltmanPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ3392009b2535
- N.LauferH.YaffeE.J.MargaliothJ.LivshinM.Ben-DavidJ.G.SchenkerEffect of bromocriptine treatment on male infertility associated with hyperprolactinemiaArch Androl61981343346
- R.WaliaA.BhansaliP.DuttaN.KhandelwalR.SialyS.BhadadaRecovery pattern of hypothalamo-pituitary-testicular axis in patients with macroprolactinomas after treatment with cabergolineIndian J Med Res1342011314319
- O.ModebeHyperprolactinemia in oligospermic Nigerian males: effect of bromocriptine treatmentInt J Fertil Menopausal Stud3919949599
- M.De RosaA.CiccarelliS.ZarrilliE.GuerraM.GaccioneA.Di Sarnoet alThe treatment with cabergoline for 24 month normalizes the quality of seminal fluid in hyperprolactinaemic malesClin Endocrinol (Oxf)642006307313
- A.ColaoG.VitaleP.CappabiancaF.BrigantiA.CiccarelliM.De Rosaet alOutcome of cabergoline treatment in men with prolactinoma: effects of a 24-month treatment on prolactin levels, tumor mass, recovery of pituitary function, and semen analysisJ Clin Endocrinol Metab89200417041711
- M.De RosaA.ColaoA.Di SarnoD.FeroneM.L.LandiS.Zarrilliet alCabergoline treatment rapidly improves gonadal function in hyperprolactinemic males: a comparison with bromocriptineEur J Endocrinol1381998286293
- A.ColaoM.De RosaF.SarnacchiaroA.Di SarnoM.L.LandiE.Iervolinoet alChronic treatment with CV 205–502 restores the gonadal function in hyperprolactinemic malesEur J Endocrinol1351996548552
- K.NishimuraK.MatsumiyaN.TsuboniwaM.YamanakaM.KogaH.Miuraet alBromocriptine for infertile males with mild hyperprolactinemia: hormonal and spermatogenic effectsArch Androl431999207213
- Mcquaid JW, Tanrikut C. Physiology of testosterone. In: Mulhall JP, Hsiao W, editors. Men’s sexual health and fertility. A Clinician’s guide. New York: Springer Science and Business Media; 2014.
- R.TsutsumiN.J.WebsterGnRH pulsatility, the pituitary response and reproductive dysfunctionEndocr J562009729737
- Hair WM, Gubbay O, Jabbour HN, Lincoln GA. Prolactin receptor expression in human testis and accessory tissues: localization and function. Mol Hum Reprod 2002;8:606–11.
- H.G.KlemckeA.G.AmadorA.BartkeHormonal regulation of testicular prolactin receptors and testosterone synthesis in golden hamstersBiol Reprod431990162168
- H.N.JabbourG.A.LincolnProlactin receptor expression in the testis of the ram: localisation, functional activation and the influence of gonadotrophinsMol Cell Endocrinol1481999151161
- M.B.WhyteS.PramodhL.SrikuganJ.A.GilbertJ.P.MiellR.A.Sherwoodet alImportance of cannulated prolactin test in the definition of hyperprolactinaemiaPituitary182015319325
- M.AgarwalA.DasA.S.SinghHigh-dose hook effect in prolactin macroadenomas: a diagnostic concernJ Hum Reprod Sci32010160161
- M.KasumS.OreskovicI.ZecD.JezekV.TomicV.Gallet alMacroprolactinemia: new insights in hyperprolactinemiaBiochem Med (Zagreb)222012171179
- C.C.LuC.J.HsiehThe importance of measuring macroprolactin in the differential diagnosis of hyperprolactinemic patientsKaohsiung J Med Sci2820129499
- S.MelmedF.F.CasanuevaA.R.HoffmanD.L.KleinbergV.M.MontoriJ.A.Schlechteet alDiagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guidelineJ Clin Endocrinol Metab962011273288
- V.S.LimS.C.KathpaliaL.A.FrohmanHyperprolactinemia and impaired pituitary response to suppression and stimulation in chronic renal failure: reversal after transplantationJ Clin Endocrinol Metab481979101107
- K.S.HonboA.J.van HerleK.A.KellettSerum prolactin levels in untreated primary hypothyroidismAm J Med641978782787
- M.E.MolitchDrugs and prolactinPituitary112008209218
- D.L.TorreA.FalorniPharmacological causes of hyperprolactinemiaTher Clin Risk Manag32007929951
- Huguet I, Clayton R. Pituitary-Hypothalamic Tumor Syndromes: Adults. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al., editors. Endotext, South Dartmouth (MA): Endotext; 2000. Available at: http://https://www.ncbi.nlm.nih.gov/books/NBK278946/. Accessed Ocober 2017.
- J.SchlechteK.DolanB.ShermanF.ChaplerA.LucianoThe natural history of untreated hyperprolactinemia: a prospective analysisJ Clin Endocrinol Metab681989412418
- Wang AT1, Mullan RJ, Lane MA, Hazem A, Prasad C, Gathaiya NW et al. Treatment of hyperprolactinemia: a systematic review and meta-analysis. Syst Rev 2012;1:33.
- Webster J, Piscitelli G, Polli A, D'Alberton A, Falsetti L, Ferrari C et al. Dose-dependent suppression of serum prolactin by cabergoline in hyperprolactinaemia: a placebo controlled, double blind, multicentre study. European Multicentre Cabergoline Dose-finding Study Group. Clin Endocrinol (Oxf) 1992; 37:534–41.
- A.ColaoA.Di SarnoM.L.LandiF.ScavuzzoP.CappabiancaR.Pivonelloet alMacroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: a prospective study in 110 patientsJ Clin Endocrinol Metab85200022472252
- J.J.PinzoneL.KatznelsonD.C.DanilaD.K.PaulerC.S.MillerA.KlibanskiPrimary medical therapy of micro- and macroprolactinomas in menJ Clin Endocrinol Metab85200030533057
- RxList. Dostinex (cabergoline); 2016. Available at: http://www.rxlist.com/dostinex-drug.htm. Accessed October 2017.
- RxList. Cycloset (bromocriptine mesylate tablets), 2017. Available at: http://https://www.rxlist.com/cycloset-drug.htm. Accessed October 2017.
- A.BarlierP.JaquetQuinagolide – a valuable treatment option for hyperprolactinaemiaEur J Endocrinol1542006187195
- A.ColaoA.Di SarnoP.CappabiancaC.Di SommaR.PivonelloG.LombardiWithdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemiaN Engl J Med349200320232033
- M.P.GillamM.E.MolitchG.LombardiA.ColaoAdvances in the treatment of prolactinomasEndocr Rev272006485534
- Brada M, Jankowska P. Radiotherapy for pituitary adenomas. Endocrinol Metab Clin North Am 2008;37:263–75, xi.
- T.W.WongT.M.JonesHyperprolactinemia and male infertilityArch Pathol Lab Med10819843539
- S.SegalH.YaffeN.LauferM.Ben-DavidMale hyperprolactinemia:effects on fertilityFertil Steril321979556561
- C.A.AdejuwonA.O.IlesanmiE.O.OdeHyperprolactinaemia as a cause of male infertility in IbadanWest Afr J Med1819991719
- G.F.GonzalesM.Garcia-HjarlesG.VelasquezHyperprolactinaemia and hyperserotoninaemia: their relationship to seminal qualityAndrologia24199295100
- J.M.Soler FernándezF.Caravaca MagariñosC.Domínguez BravoHerreraPuertoCorrelation of serum prolactin, sperm count and motility. Prevalence of hyperprolactinemia in the infertile maleJ Arch Esp Urol431990891895
- F.LottiG.CoronaE.MaseroliM.RossiA.SilveriiS.Degl'innocentiet alClinical implications of measuring prolactin levels in males of infertile couplesAndrology12013764771
- H.OkadaT.IwamotoH.FujiokaT.ShirakawaN.TatsumiM.Kanzakiet alHyperprolactinaemia among infertile patients and its effect on sperm functionsAndrologia281996197202
- S.MićićR.DotlićV.IlićO.GenbacevHormone profile in hyperprolactinemic infertile menArch Androl151985123128