Abstract
Objective To discuss the role, reliability and limitations of the semen analysis in the evaluation of fertility with reference to the World Health Organization (WHO) fifth edition guidelines, with semen analysis reference values published in 2010. We also discuss the limitations of using a single threshold value to distinguish ‘abnormal’ and ‘normal’ parameters.
Methods The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to search the MEDLINE, EMBASE, and the Cochrane electronic database for articles discussing the effectiveness of semen analysis.
Results Limitations affecting the reliability of semen analysis as a predictor of fertility were found. These include: the lack of consideration of the female factor, the vaguely defined threshold values, and the intra-individual variation in semen parameters.
Conclusions Impaired semen parameters alone cannot be used to predict fertility as these men still have a chance of being fertile, except when a man has azoospermia, necrospermia or globozoospermia.
Introduction
Semen analyses have been the test of choice for assessing the male partner in an infertile couple. Studies linking quality of the ejaculate and fertility have been around since the 1930s [Citation1]. In 1980, the WHO recruited a team of physicians and scientists to publish a manual describing in detail what the normal semen parameters of a fertile man should be and how to analyse semen in the laboratory. Since then, they have updated the manual four times, with the latest being in 2010 [Citation2]. Although analysing semen samples can provide valuable information about the fertility of the male in certain situations, it does have several limitations [Citation3]. The goal of the present review is to discuss the role, reliability and limitations of semen analyses in the evaluation of infertility, as well as the disadvantages of using a single threshold value to distinguish abnormal and normal parameters.
What is a semen analysis?
Semen consists of two components: the spermatozoa made by the seminiferous tubules of the testis, and the seminal fluid produced by the accessory glands that nourishes sperm and has a role in interacting with the female reproductive tract to influence fertility [Citation4]. These components are reflected in the semen analysis by the sperm count, which reflects the number of spermatozoa in the semen sample; and the volume of the semen, which reflects the amount of seminal fluid produced [Citation2]. Sperm motility is defined as the percentage of sperm that show signs of movement, whilst the sperm morphology is the percentage of sperm that appears to have a normal cellular structure. Sperm vitality is defined as the percentage of sperm that are viable in the sample.
A semen sample is collected by masturbation after an abstinence period of 2–7 days, preferably near the laboratory to limit the time between collection and analysis. The physical characteristics of the semen sample, such as the volume, pH, colour, liquefaction and viscosity is measured, and the sample is then evaluated under a microscope to determine the motility, vitality, concentration, and morphology [Citation5]. The values obtained are compared to the reference values determined by the WHO manual. The reference ranges for semen characteristics are given in .
Table 1 Semen analyses lower reference limits defined by the WHO laboratory manual for the examination and processing of human semen in 2010.
Studies have shown that the total motile sperm count (volume × concentration × motility) has been the most predictive factor in determining fertility compared to volume, concentration, and motility individually [Citation6].
Determining normal semen parameters
The reference values for the semen parameters established in the 2010 WHO manual were described in a report by Cooper et al. [Citation7] in 2010. The study analysed the semen analysis data from 1953 men from five studies across three continents. The 1953 men included in the study had got their partner pregnant in ≤12 months ensuring that all these men were fertile. After the semen analysis the data were analysed, the 5th centile was calculated and it was used as the lower reference limit in the fifth edition manual published by the WHO. The 5th centile for reference ranges was used to match what is widely accepted in clinical chemistry, which states that 95% of the data should be included in the reference interval.
There are many limitations with the study, some of which were discussed by the authors themselves. The lack of an equal population distribution means that some areas of the world are over-represented, whilst others are under-represented [Citation7]. For example, only 10% of the population involved in the study was from the southern hemisphere, and most of the included population was from Europe [Citation5].
Another limitation is that only fertile couples were included in the evaluation of normal values. Whilst the WHO study did account for this by comparing it to a normal ‘unscreened’ group that represents the general population, they never compared their values to that of infertile men. Because of this, it is difficult to accurately predict fertility with the WHO reference values [Citation7].
Methods
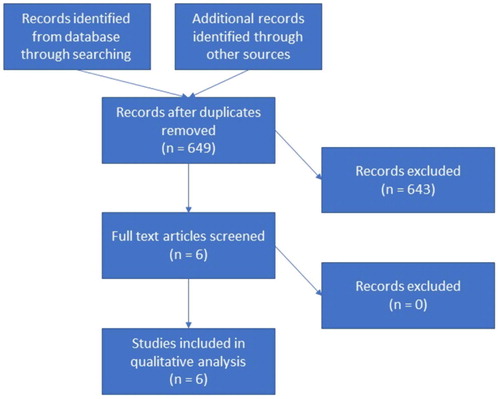
We reviewed the databases of MEDLINE, EMBASE, and the Cochrane Library as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines on 15 August, 2017 ( ) [Citation8]. The search was conducted with the following keywords: ‘semen analysis’, ‘World Health Organization’ and ‘infertility’. Articles were evaluated based on their title or abstract and were included if they discussed the reliability or the limitations of semen analysis. Articles were excluded if they were not written in English.
Results
We identified 649 articles across the three databases and after reviewing the titles and abstract for the articles, we were left with six articles that met the inclusion criteria ( ) [Citation3,Citation5,Citation6,Citation9Citation[10]–Citation11]. Two of the articles were review articles, another two were prospective cohort studies, and the other two were case–control studies. We reviewed each article and determined the biggest limitations to be the lack of consideration of the female factor, the vaguely defined threshold values, and the intra-individual variation in semen parameters. These will be discussed below.
Table 2 List of studies included in the review with comments.
Discussion
Limitations of semen analyses
The female factor
About half of the infertile couples have a male component of infertility and only 30% of infertility in couples is due to male factors alone [Citation12]. The Center for Disease Control (CDC) reports that 12.1% of females aged 15–44 years in America have impaired fecundity [Citation13]. Age is a strong factor that affects fertility in females. This is mainly due to a decrease in oocyte number and quality with age [Citation14,Citation15]. An example of the decrease in quality is the increased incidence of autosomal trisomies with age that has been proven in a variety of study populations [Citation16,Citation17].
The WHO fifth edition manual used studies that did not emphasise female age. Most of the studies only included pregnant women and only had age-limiting inclusion criteria for men; the studies that did provide information about the female partner had a mean female age of ∼29 years, and showed a low percentage of females aged >35 years [Citation11,Citation18].
Female age must be considered when talking about a couple’s fertility. Males with poor quality sperm could conceive when their relative subfertility is compensated by a young female with a high probability of conception [Citation19]. That same man may experience problems with conception if his partner is a 45-year-old woman [Citation14,Citation15]. In this case, poor results in a semen analysis could skew physicians to believe that the male is the cause of the couple’s infertility. It is important to remember that infertility itself is a couple’s issue and it must be treated as such.
Re-defining the threshold values
There is a large controversy in the value of the fixed thresholds with semen analyses in the evaluation of fertility. The use of the 5th centile in the WHO manual means that, out of the 1953 fertile men, 5% of them had semen parameters below the lower reference limit for normal. Large variations in semen analysis results are seen in the fertile population and whilst there is an increased likelihood of conception with an increased sperm count, males with lower sperm counts can still conceive.
A 1998 study by Bonde et al. [Citation6] investigated 430 first-pregnancy couples and their results showed that ∼65% of the males with sperm concentrations >20 million/mL conceived and 36% of males with concentrations below that conceived. This shows that it is impossible to determine what a low sperm count means for an individual man, as there are too many other factors that affect fertility. The reverse is also true in that a man with a normal sperm count may not be able to conceive a child due to factors such as sperm aneuploidy and high sperm DNA fragmentation.
Guzick et al. [Citation9] studied the differences in semen parameters between fertile and infertile couples after excluding the female factor by fertility evaluation. They discussed that it is more appropriate to separate semen measurements into three groups: fertile, indeterminate, and subfertile, rather than using a single reference value to delineate normal and abnormal values, as done in the current WHO guidelines. The reference values for these three groups are shown in [Citation9].
Table 3 ‘Fertile’, ‘indeterminate’, and ‘subfertile’ semen measurements derived from Classification-and-regression-tree analysis by Guzick et al. 2001 [Citation9].
There is no doubt that the likelihood of infertility increases as the percentage of sperm with normal morphology, motility, and concentration decreases [Citation9,Citation11,Citation18]. Both studies described above show a marked excess of fertile men with values in the fertile range; and a corresponding excess of infertile men with values in the subfertile range. However, there is an overlap in between these groups, where both fertile and infertile men are distributed in almost equal proportions. They described these values as the ‘indeterminate’ range [Citation6,Citation9]. They concluded that the three-group classification system would be more clinically meaningful for their data, given that semen measurements progress biologically in a continuous variation. This is in contrast with the categorical, discontinuous fashion that separates normal and abnormal semen values in the WHO guidelines [Citation2,Citation9].
In conclusion, caution should be undertaken whenever interpreting subfertile sperm measurements as these are not conclusive of infertility. The same goes for men whose sperm counts are above the reference range set by the WHO manual, as these men could have parameters in the indeterminate range where a significant portion of the group is infertile.
Intra-individual variability
Apart from the study by Leushuis et al. [Citation10], many other studies also described fluctuations of semen measurement values within the same individual [Citation20Citation[21]Citation[22]–Citation23]. Alvarez et al. [Citation24] discussed that sperm concentration shows the greatest intra-individual variation followed by other parameters such as: sperm count, morphology, motility, and semen volume. On the other hand, the parameters that showed the best homeostatic regulation with the least variation within an individual is sperm vitality and total motility [Citation23,Citation24]. All these variations are probably due to the differences in sperm production in the normal germinal epithelium [Citation24].
The process of spermatogenesis is initiated and highly regulated by hormonal controls in the hypothalamus. GnRH from the hypothalamus triggers the release of FSH and LH, which in turn initiates spermatogenesis in the testes. GnRH stimulation is regulated by different rhythmicities in the body, one of which is the circadian rhythm with a daily peak in GnRH production during the early morning. Besides that, the pulsatile rhythm also gives a peak output of GnRH every 90–120 min [Citation25]. Thus, we can imagine that due to the control and regulation of GnRH secretion, this will in turn influence the process of spermatogenesis resulting in the daily variations of semen parameters.
Furthermore, the Cagnacci et al. [Citation26] study demonstrates the diurnal variation of semen quality in human males contributing to the reported variability in semen parameters. Their data document that specimens collected in the afternoon showed higher numbers and concentration of spermatozoa compared to those collected in the morning. This was also true for the progressive linear motility of spermatozoa.
In another study by Sebastian-Gambaro et al. [Citation22], it is hypothesised that the intra-individual variations are due to the random and rhythmic fluctuations of the quantity values around a virtual homeostatic set point.
Apart from the day-to-day variations, some studies have also suggested that semen analysis parameters vary according to seasons. Zhang et al. [Citation27] discussed that semen quality in the midsummer was found to be significantly lower when compared to other periods of the year. In other studies, there was a gradual decrease in the fast forward motility and sperm concentration from spring towards autumn (fall) with recovery during winter. Furthermore, the percentage of sperm with normal morphology was found to be higher in the winter and spring compared to the summer. These seasonal sperm patterns seem to be a circannual-rhythmic phenomenon and adds to the intra-individual variation observed [Citation28,Citation29].
Having large intra-individual variations weakens the strength of using a single semen analysis to assess male fertility, as it means that a man might have lower semen analysis parameters as a result of normal variation rather than problems with fertility, even after the abstinence period is controlled. At least two semen analyses are recommended to properly evaluate male fertility because of these fluctuations [Citation30]. However, Leushuis et al. [Citation10] showed that adding a second test does not improve the reliability of the prediction of natural conception.
In conclusion, conventional reference values for semen parameters have little diagnostic value for infertility due to their marked individuality, although it may be useful for assessing differences in serial results in the same individual, especially in terms of sperm motility and vitality [Citation24].
Absolute predictors of fertility on semen analysis
Despite all the cases in which the semen analysis may not be a good predictor of fertility, there are some conditions that can lead to infertility that will be reflected in a semen analysis. These are the extremes in the values when there is azoospermia, severe asthenospermia (0% motility), or globozoospermia [Citation3]. Men with these findings on semen analysis are guaranteed to be infertile, and they are the only cases in which semen analyses can predict infertility with absolute certainty.
Azoospermia
Azoospermia is defined as the complete absence of sperm from the ejaculate [Citation31]. It is diagnosed based on the absence of spermatozoa on microscopic analysis after 15 min of maximum speed centrifugation of complete semen specimens. The semen analysis should be performed following the 2010 WHO guidelines, and at least two semen samples obtained more than 2 weeks apart should be examined [Citation2,Citation30].
Azoospermia can be classified into two broad categories: obstructive and non-obstructive. In more than 90% of cases, the patient’s history, physical examination and hormonal analysis (FSH, testosterone) will be able to define the cause of azoospermia [Citation31]. However, a testicular biopsy is the only definitive way to diagnose azoospermia [Citation32].
In obstructive azoospermia, there is some form of obstruction along the reproductive tract, obstructing passage of sperm from the testis into the ejaculate. These may be due to congenital causes, such as congenital bilateral absence of the vas deferens (CBAVD) in the case of cystic fibrosis; or epididymal obstruction seen in Young’s syndrome [Citation33,Citation34]. Obstructive azoospermia can also be acquired from previous infections, trauma, vasectomies, and iatrogenic injuries.
On the other hand, non-obstructive azoospermia is mostly due to defects in the production of spermatozoa. These may be caused by hormonal irregularities and endocrine pathologies such as: hypogonadotrophic hypogonadism (HGH), usually due to pituitary lesions; Kallmann syndrome; and anabolic steroid use. Non-obstructive azoospermia may also be due to intrinsic disorders of the testes affecting spermatogenesis such as: varicoceles, cryptorchidism, testicular torsion, gonadotoxins, and genetic abnormalities. These intrinsic abnormalities constitute 65–80% of cases for male infertility [Citation32].
Asthenospermia
The most common reason for severe asthenospermia (0% motility) is epididymal or ejaculatory duct obstruction. Individuals with genital tract obstruction appear to be at an increased risk for anti-sperm antibodies (ASA), which are autoimmune antibodies against sperm cells. Elevated ASA titres have been found in 81% of men with obstruction as compared to 10% of men with other causes of infertility [Citation35Citation[36]–Citation37]. Once these antibodies are generated due to inoculation of the sperm antigens to the immune system, ASA will impair sperm motility, passage through the female reproductive tract, and will also affect the capacity for sperm to properly interact with the oocyte during fertilisation [Citation38].
When >50% of sperm are bound to antibodies, the probability of sufficient numbers of sperm penetrating the cervical mucus is significantly reduced [Citation39]. Experimental studies suggest that the Fc region of immunoglobulin A (IgA) binds receptors within the mucus, impairing forward progression of the sperm [Citation40].
One of the most prominent genetic abnormalities affecting the motility of the flagellum is primary ciliary dyskinesia (PCD), or immotile cilia syndrome. It is usually associated with recurrent sinopulmonary infections, situs inversus, and male infertility (with asthenospermia and oligospermia).
Other risk factors for ASA production include prior infections, trauma or reproductive tract surgery, e.g. vasovasostomy or vasoepididymostomy. According to the AUA guidelines, ASA testing can be considered given the setting of isolated asthenospermia with normal sperm concentration [Citation41].
Globozoospermia
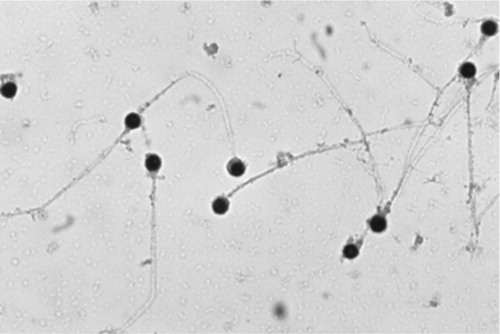
Another absolute predictor for male infertility is the detection of globozoospermia on semen analysis. It is a rare but severe form of teratozoospermia, characterised by the presence of round-headed spermatozoa lacking an acrosome [Citation42]. The acrosome contains the digestive enzyme, acrosin, which is essential for binding and penetration of the zona pellucida of the ovum. It also facilitates cervical mucus penetration and intrauterine sperm migration. It also participates in chromatin decondensation in the oocyte [Citation43]. Considering these factors, we can understand how globozoospermic cells have difficulties adhering and fusing with the oocyte membrane, ultimately causing infertility. A microscopic image of globozoospermic spermatozoa can be seen in [Citation44].
Fig. 2 Globozoospermic spermatozoa seen under a microscope. A condition where the acrosomal caps are absent and the sperm heads becomes globular. From Jequier 2011 [Citation44] – Fig. 8.2, with permission.
In summary, abnormalities in all the three semen parameters (sperm concentration, motility and morphology) can indicate subfertility but only extreme losses can accurately predict infertility.
Conclusion
A semen analysis is only a gross estimation of fertility but it is, unfortunately, the best test we have. Semen analysis can predict fertility in men with azoospermia, severe asthenospermia and globozoospermia. In other cases, semen analyses have a limited role in the evaluation of infertility because female factors can influence fecundity in most couples. It is important to realise that all men who were included in the WHO fifth edition were fertile. Lower reference limit threshold points for semen parameters at the 5th percentile should be used as a reference for counselling couples and not be used to label men as ‘fertile’ or ‘infertile’.
Contributions
(I) Conception and design: Ranjith Ramasamy; (II) Administrative support: None; (III) Provision of study material or patients: None; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Conflict of interest
None.
Source of Funding
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- D.MacomberM.B.SandersThe spermatozoa countN Engl J Med2001929984987
- World Health Organization Department of Reproductive Health and Research. WHO laboratory manual for the examination and processing of human semen, fifth edition. WHO 2010. Available at: http://www.who.int/reproductivehealth/publications/infertility/9789241547789/en/. Accessed October 2017.
- C.WangR.S.SwerdloffLimitations of semen analysis as a test of male fertility and anticipated needs from newer testsFertil Steril102201415021507
- J.E.SchjenkenS.A.RobertsonSeminal fluid signalling in the female reproductive tract: implications for reproductive success and offspring healthAdv Exp Med Biol8682015127158
- S.C.EstevesA.ZiniN.AzizJ.G.AlvarezE.S.SabaneghJrA.AgarwalCritical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile menUrology7920121622
- J.P.BondeE.ErnstT.K.JensenN.H.HjollundH.KolstadT.B.Henriksenet alRelation between semen quality and fertility: a population-based study of 430 first-pregnancy plannersLancet352199811721177
- T.G.CooperE.NoonanS.von EckardsteinJ.AugerH.W.BakerH.M.Behreet alWorld Health Organization reference values for human semen characteristicsHum Reprod Update162010231245
- D.MoherA.LiberatiJ.TetzlaffD.G.AltmanPGroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementInt J Surg82010336341
- D.S.GuzickJ.W.OverstreetP.Factor-LitvakC.K.BrazilS.T.NakajimaC.Coutifariset alSperm morphology, motility, and concentration in fertile and infertile menN Engl J Med345200113881393
- E.LeushuisJ.W.van der SteegP.SteuresS.ReppingP.M.BossuytB.W.Molet alSemen analysis and prediction of natural conceptionHum Reprod29201413601367
- R.SlamaF.EustacheB.DucotT.K.JensenN.JorgensenA.Horteet alTime to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European citiesHum Reprod172002503515
- V.M.Brugh3rdL.I.LipshultzMale factor infertility: evaluation and managementMed Clin North Am882004367385
- Centres for Disease Control and Prevention. National Center for Health Statistics – Infertility Statistics, 2016. Available at: https://www.cdc.gov/nchs/fastats/infertility.htm. Accessed October 2017.
- N.M.CrawfordA.Z.SteinerAge-related infertilityObstet Gynecol Clin North Am4220151525
- American College of Obstetricians and Gynecologists Committee onGynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589Fertil Steril1012014633634
- M.LoaneJ.K.MorrisM.C.AddorL.ArriolaJ.BuddB.Dorayet alTwenty-year trends in the prevalence of Down syndrome and other trisomies in Europe: impact of maternal age and prenatal screeningEur J Hum Genet2120132733
- K.JaruthamsophonH.SriplungC.CharalsawadiP.LimprasertMaternal age-specific rates for trisomy 21 and common autosomal trisomies in fetuses from a single diagnostic center in ThailandPLoS ONE112016e0165859
- T.K.JensenR.SlamaB.DucotJ.SuominenE.H.CawoodA.G.Andersenet alRegional differences in waiting time to pregnancy among fertile couples from four European citiesHum Reprod16200126972704
- Jungwirth A. Diemer T, Dohle GR, Giwercwan A, Kopa Z, Kraucz C, et al. Guidelines on male infertility. European Association of Urology, 2015. Available at: https://uroweb.org/wp-content/uploads/17-Male-Infertility_LR1.pdf. Accessed October 2017.
- C.MallidisE.J.HowardH.W.BakerVariation of semen quality in normal menInt J Androl14199199107
- M.L.PolandK.S.MoghissiP.T.GiblinJ.W.AgerJ.M.OlsonVariation of semen measures within normal menFertil Steril441985396400
- M.A.Sebastian-GambaroF.J.Liron-HernandezX.Fuentes-ArderiuIntra- and inter-individual biological variability data bankEur J Clin Chem Clin Biochem351997845852
- U.A.KnuthJ.KuhneM.Bals-PratschE.NieschlagIntra-individual variation of sperm velocity, linearity, lateral head displacement and beat frequency in healthy volunteersAndrologia201988243248
- C.AlvarezJ.A.CastillaL.MartinezJ.P.RamirezF.VergaraJ.J.GaforioBiological variation of seminal parameters in healthy subjectsHum Reprod18200320822088
- R.K.SharmaA.AgarwalSpermatogenesis: an overview2011SpringerNew York, NY
- A.CagnacciN.MaxiaA.VolpeDiurnal variation of semen quality in human malesHum Reprod141999106109
- X.Z.ZhangJ.H.LiuH.Q.ShengH.J.WuY.WuK.S.Yaoet alSeasonal variation in semen quality in ChinaAndrology12013639643
- R.OzelciS.YilmazB.DilbazF.AkpinarD.Akdag CirikS.Dilbazet alSeasonal variation of human sperm cells among 4,422 semen samples: a retrospective study in TurkeySyst Biol Reprod Med622016379386
- E.LevitasE.LunenfeldN.WeiszM.FrigerI.Har-VardiSeasonal variations of human sperm cells among 6455 semen samples: a plausible explanation of a seasonal birth patternAm J Obstet Gynecol2082013406 406.e1–6
- J.A.CastillaC.AlvarezJ.AguilarC.Gonzalez-VareaM.C.GonzalvoL.MartinezInfluence of analytical and biological variation on the clinical interpretation of seminal parametersHum Reprod212006847851
- P.N.SchlegelCauses of azoospermia and their managementReprod Fertil Dev162004561572
- M.CocuzzaC.AlvarengaR.PaganiThe epidemiology and etiology of azoospermiaClinics (Sao Paulo)68Suppl. 120131526
- T.McCallumJ.MilunskyR.MunarrizR.CarsonH.Sadeghi-NejadR.OatesUnilateral renal agenesis associated with congenital bilateral absence of the vas deferens: phenotypic findings and genetic considerationsHum Reprod162001282288
- D.J.HandelsmanA.J.ConwayL.M.BoylanJ.R.TurtleYoung's syndrome. Obstructive azoospermia and chronic sinopulmonary infectionsN Engl J Med310198439
- P.J.TurekL.I.LipshultzImmunologic infertilityUrol Clin North Am211994447468
- S.M.GirgisE.M.EkladiousR.IskanderR.El-DakhlyF.N.GirgisSperm antibodies in serum and semen in men with bilateral congenital absence of the vas deferensArch Androl81982301305
- W.F.HendryJ.M.ParslowJ.StedronskaD.M.WallaceThe diagnosis of unilateral testicular obstruction in subfertile malesBr J Urol541982774779
- C.S.CroppW.D.SchlaffAntisperm antibodiesArch Immunol Ther Exp (Warsz)3819903146
- B.FjallbrantCervical mucus penetration by human spermatozoa treated with anti-spermatozoal antibodies from rabbit and manActa Obstet Gynecol Scand4819697184
- S.JagerJ.KremerImmunological aspects of male infertilityAnn Biol Clin (Paris)451987340345
- Jarow J, Sigman M, Kolettis PN, Lipshultz LR, McClure RD, Nangia AK, et al. Optimal evaluation of the infertile male. American Urological Association; 2011. Available at: http://www.auanet.org/guidelines/male-infertility-optimal-evaluation-(reviewed-and-validity-confirmed-2011). Accessed September 2017.
- A.H.DamI.FeenstraJ.R.WestphalL.RamosR.J.van GoldeJ.A.KremerGlobozoospermia revisitedHum Reprod Update1320076375
- W.B.SchillE.Topfer-PetersenE.HeisslerThe sperm acrosome: functional and clinical aspectsHum Reprod31988139145
- A.M.JequierMale infertility: a clinical guide (Cambridge Clinical Guides)2nd ed.2011Cambridge University PressCambridge6267