Abstract
Objective To summarise the latest evidence on the role of sperm DNA fragmentation (SDF) in male factor infertility, as SDF has been emerging as a valuable tool for male infertility evaluation.
Methods A search of PubMed was conducted using the keywords ‘sperm DNA fragmentation’ and ‘male infertility’. Studies in languages other than English were excluded. All identified studies were screened and clinical studies in humans were included.
Results In all, 150 articles were included for analysis. Current evidence supports the association between high SDF and poor reproductive outcomes for natural conception and intrauterine insemination. Although the relationship between high SDF and in vitro fertilisation and intracytoplasmic sperm injection is less clear, the negative implication of high SDF on pregnancy loss is evident. Various treatment strategies have been attempted with varying success. The predictive value of SDF testing on outcomes of natural pregnancy and assisted reproduction illustrates its value in clinical practice.
Conclusion The significant role of SDF in male factor infertility is supported by current evidence. The beneficial role of SDF testing in selection of varicocelectomy candidates, evaluation of patients with unexplained infertility and recurrent pregnancy loss, selection of the most appropriate assisted reproductive technique with highest success rate for infertile couples, and assessment of infertile men with modifiable lifestyle factors or gonadotoxin exposure has been recently proposed.
Abbreviations:
- ART
- assisted reproductive technology
- ASRM
- American Society for Reproductive Medicine
- Comet
- single cell gel electrophoresis assay
- EAU
- European Association of Urology
- ICSI
- intracytoplasmic sperm injection
- IMSI
- intracytoplasmic morphologically selected sperm injection
- IUI
- intrauterine insemination
- IVF
- in vitro fertilisation
- MACS
- magnetic cell sorting
- PICSI
- physiological ICSI with hyaluronic acid binding assay
- OR
- odds ratio
- ROS
- reactive oxygen species
- RR
- relative risk
- SCD
- sperm chromatin dispersion
- SCSA
- sperm chromatin structure assay
- SDF
- sperm DNA fragmentation
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick end labelling
Introduction
About 15% of couples of reproductive age are affected by infertility, with male factors contributing up to 50% of the reduced fertility [Citation1]. However, since the advent of intracytoplasmic sperm injection (ICSI), little attention has been given to the evaluation of infertile men. Conventional semen analysis remained the only routine diagnostic test for male infertility, despite the lack of capability in discriminating infertile from fertile men and up to 40% of infertile men have semen parameters within normal reference ranges [Citation2]. Even with the rapid development of assisted reproductive technology (ART) during the last few decades, the live-birth rate does not exceed 30% [Citation3]. Resolving the problem of male infertility is the rational approach to improve treatment outcomes of infertile couples rather than bypassing the male factors with ART.
There is a need to search for additional diagnostic tools to improve prediction of fertility and direct management decisions of infertile couples. An understanding of male infertility at the molecular level and the recognition of sperm DNA integrity has revived interest in sperm function tests in recent years. Emerging evidence on the role of sperm DNA integrity on reproductive outcomes and development of sperm DNA fragmentation (SDF) assays opens a new horizon in clinical andrology. Although the routine use of SDF testing in the evaluation of infertile men is generally not supported, the value of the test has been acknowledged in the latest AUA and European Association of Urology (EAU) guidelines [Citation4,Citation5].
The present review summarises the role of SDF in male infertility. Firstly, normal sperm chromatin structure and the causative mechanisms of SDF are briefly introduced. Currently available SDF assays are also discussed. Secondly, the implications of SDF on natural pregnancy and ART outcomes are presented. The possible genetic consequences and birth defects in offspring are put forward. Finally, treatment options for high SDF and clinical application of SDF tests are proposed.
Materials and methods
The PubMed database was searched from time of inception to October 2017. The search was limited to studies published in English and only human studies were included. The search terms were ‘sperm DNA fragmentation’ and ‘male infertility’. The two keywords were combined with ‘AND’ to capture all citations that were relevant to our research question.
Studies were selected if male patients with primary or secondary infertility were included in the target population. The inclusion criteria for this review were reporting of clinical outcome parameters including fertilisation, pregnancy, birth, and miscarriage rates. Studies involving interventions that did not alter clinical outcomes were excluded. Relevant information was extracted from all studies that fulfilled the selection criteria. The most recent or the most complete publication was used in cases of duplicate publications.
Results
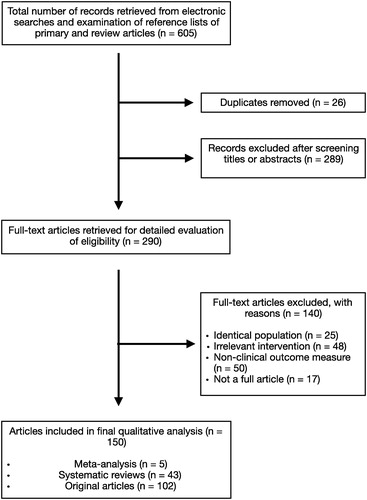
In all, 605 studies were initially retrieved from the database using the search strategy described above. Review of the titles and abstracts of all studies indicated 26 duplicate and 289 irrelevant studies. The remaining 290 studies were scrutinised by the authors. Of these, 140 studies were excluded due to various reasons as listed in . Eventually, 150 studies met the inclusion criteria and were included in the review.
Discussion
SDF
Sperm are specifically designed for the transmission of a complete haploid genome to the ovum that will ultimately constitute a new individual. The success of the process partly relies on the compaction of genetic material adjusting to the extremely limited volume of the sperm nucleus. Protamination represents the unique process in which histones are removed and replaced by positively charged protamines forming tight toroidal complexes during the condensation process. Emerging evidence supports the importance of chromatin organisation during fertilisation and early embryo development [Citation6]. In fact, a certain degree of DNA breaks are present in sperm from fertile men and the level varies from one sperm to another [Citation7]. It is thought that controlled DNA nicking during DNA compaction is essential to relieve the stress in the molecule. However, infertile men usually have a larger proportion of sperm with higher levels of fragmented DNA than fertile men.
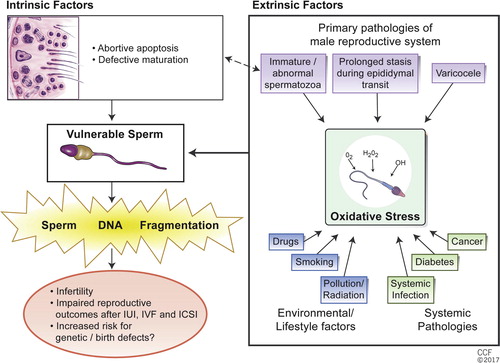
Both intrinsic and extrinsic factors are involved in the pathogenesis of fragmented DNA. Poor chromatin structure renders the sperm vulnerable to DNA damage in the face of extrinsic factors. The lack of a DNA repair mechanism in sperm also explains its susceptibility to DNA fragmentation. Abortive apoptosis [Citation8] and defective maturation [Citation9] theories were proposed to explain the intrinsic factors in the generation of SDF in testicular sperm. However, there is evidence showing that there is more DNA fragmentation in epididymal and ejaculated sperm than in testicular sperm, suggesting extrinsic factors being more significant in most patients [Citation10]. Recently, oxidative stress has been identified as an important extrinsic cause of SDF [Citation11]. The presence of a large amount of polyunsaturated fatty acids in the plasma membrane makes sperm particularly susceptible to oxidative stress-mediated damage [Citation12]. A supraphysiological level of reactive oxygen species (ROS) overwhelms the protective antioxidant system and results in sperm DNA strand breaks [Citation13]. The close relationship between efficiency of sperm chromatin protamination and the degree of oxidative DNA damage as the causes of SDF is summarised in .
Fig. 2 Causes of SDF. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography ©2017. All Rights Reserved.
Following the increasing interest in the association between SDF and reduced fertility, the advancement in molecular biology and SDF assays in the 1980s and 1990s has hastened the research in the area. Currently, eight methods to assess SDF () are clinically available, which are generally classified into two types: direct and indirect. Whilst direct tests measure the extent of sperm DNA damage by using probes and dyes, indirect tests assess the susceptibility of DNA to denaturation that occurs more commonly in fragmented DNA [Citation14]. Amongst the SDF assays, the sperm chromatin structure assay (SCSA), terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL), sperm chromatin dispersion (SCD) and single cell gel electrophoresis assay (Comet) have standardised protocols and represent the mostly widely used tests [Citation15].
Table 1 SDF testing methods.
Although the results from different SDF assays are generally not comparable due to the different aspects of SDF measured [Citation16], the tests are interrelated by commonly reflecting the properties of sperm DNA and may indicate a common origin of damage [Citation17]. Indeed, moderate correlations, with coefficients ranging from 0.3 to 0.7, have been reported amongst various SDF tests including SCSA, SCD and TUNEL assays [Citation18]. Moreover, the predictive value of SDF in both natural pregnancy and ART outcomes has been consistently reported from various centres using different testing methods in a wide range of patients [Citation19]. It appears that a standardised protocol and specimen collection for SDF assays in a specialised andrology laboratory with good quality control is critical in generating a clinically useful result, irrespective of the different testing methods used [Citation15]. The lack of a clear threshold value for SDF assays is often considered as another pitfall, deterring the wider application of the test. However, fertility potential should be conceptualised in terms of probability rather than a bimodal parameter. The quest for a clear threshold of a diagnostic test in the context of complex human reproductive system is probably an oversimplification. The coexistence of factors from both partners in an infertile couple cannot be accurately assessed by a single laboratory test on either partner. Whilst a clear threshold value is preferred for a scientific study, it is reasonable to adjust the acceptable level of SDF according to a specific clinical scenario in accordance to other confounders [Citation20].
Studies on the possible correlation between SDF and conventional semen parameters yielded ambiguous conclusions. On one hand, a negative association between SDF and morphologically normal spermatozoa has been reported. On the other hand, the fact that sperm with high SDF can have normal motility and morphology suggests additional prognostic value of the assessment [Citation21]. In fact, the value of SDF as an independent attribute of semen quality in additional to conventional semen analysis has recently been supported [Citation22]. A higher level of SDF is also found in men with abnormal semen parameters and normozoospermic partners of infertile couples [Citation23]. SDF test results reflect overall sperm quality to a certain extent and are complementary to semen analysis, but more significant and distinct than conventional semen parameters.
Implications of SDF on reproductive outcomes
Natural pregnancy
The number of reports on the relationship between SDF and natural pregnancy may seem scarce compared to reports on ART outcomes [Citation19], but good quality data are not lacking. Time-to-pregnancy, which is an excellent endpoint in assessment of fertility potential, has been reported. The Danish First Pregnancy Planner study provided solid evidence by illustrating the correlation between infertility and an SDF index of >30% in an unselected population of unknown fertility capability. A high proportion of sperm exhibiting SDF was associated with a longer time to achieve natural pregnancy, in addition to lower fertility potential, compared to low SDF [Citation24]. In the Longitudinal Investigation of Fertility and the Environment study, which enrolled ∼500 couples with no infertility history discontinuing contraception for the purpose of becoming pregnant, SDF was associated with fecundity [Citation25]. A meta-analysis involving three studies and 616 couples suggested high SDF, determined by SCSA, was associated with failure to achieve natural pregnancy with an odds ratio (OR) of 7.01 (95% CI 3.68, 13.36) [Citation26].
Intrauterine insemination (IUI)
The association between high SDF and poor IUI outcomes is not without debate. The decline in the use of IUI in many fertility centres worldwide limits data acquisition. In one study, an SDF index of >30% by SCSA was a predictor for decreased pregnancy and delivery rates after IUI with an OR of 9.9 (95% CI 2.37, 41.51) [Citation27]. Insemination of >12% TUNEL-positive spermatozoa resulted in no pregnancy in another study [Citation28]. A recent study also suggested an SDF index of >27% by SCSA has a negative impact on the IUI pregnancy rate [Citation29].
In vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI)
The relationship between SDF and pregnancy rates after IVF has been more extensively studied. Notwithstanding, the interpretation of results is limited by the heterogeneity in study populations, SDF assays, protocols, and thresholds. Furthermore, many of the studies analysed both IVF and ICSI patients as a single entity, despite the difference between the techniques.
Earlier systematic reviews have reported a modest relationship between SDF and pregnancy rates with IVF. Lower pregnancy rates in patients with high SDF with a combined OR of 1.57 (95% CI 1.18, 2.07) was observed by evaluating nine IVF studies (six using TUNEL and three SCSA) [Citation30]. Likewise, 553 patients who underwent conventional IVF were studied in another review. A statistically significant association between SDF measured by TUNEL, SCSA and Comet with an OR of 1.27 (95% CI 1.05, 1.52; P = 0.01) was reported [Citation31].
In contrast, compelling evidence suggests that SDF has a negligible effect on ICSI outcome measures. A systematic review failed to find a significant association between SDF and ICSI pregnancy rates with a combined OR of 1.14 (95% CI 0.86, 1.54) [Citation29]. Another meta-analysis included 16 cohort studies, with 3106 couples, and reported a lower pregnancy rate in the context of high SDF only in patients undergoing IVF (OR 0.66, 95% CI 0.48, 0.90; P = 0.008) but not ICSI (OR 0.94, 95% CI 0.70, 1.25) [Citation32].
Controversies remain as demonstrated by more recent systematic reviews and meta-analyses. In a recent meta-analysis, 56 studies were included and classified into IVF (16 studies), ICSI (24 studies), and mixed IVF/ICSI (16 studies) [Citation33]. The authors concluded that SDF predicts poor clinical pregnancy rates for both IVF (OR 1.65, 95% CI 1.34, 2.04; P < 0.001) and ICSI (OR 1.31, 95% CI 1.08, 1.59; P < 0.007) [Citation33]. Another systematic review and meta-analysis, on the other hand, reported a fair to poor predictive value of various SDF assays in the prediction of pregnancy after IVF or ICSI. All tests generally showed higher sensitivity and lower specificity [Citation34]. However, both meta-analyses were limited by the high study heterogeneity and poorly controlled female factors [Citation33,Citation34].
Importantly, clinical and ongoing pregnancy rates are less relevant outcomes. A recent systematic review and meta-analysis sought to examine the effect of SDF on live-birth rates in IVF and ICSI. Six observational studies (three using SCSA, two TUNEL, and one Comet) and 998 couples were identified. The meta-analysis showed that couples whose male partners had low SDF achieved higher live-birth rates after IVF (relative risk [RR] 1.27, 95% CI 1.05, 1.52) and ICSI (RR 1.11, 95% CI 1.00, 1.23). Further subgroup analysis showed that the impact of SDF on live-birth rates amplified significantly in IVF (RR 2.76, 95% CI 1.59, 4.80; P < 0.001) but became insignificant in ICSI (RR 1.08, 95% CI 0.39, 2.96) when female factors were controlled for [Citation35]. This result also signifies the potential role of ICSI in the treatment of men with high SDF.
The difference in outcome between conventional IVF and ICSI cycles may be explained by technical differences between the two techniques. In IVF, both gametes are subjected to prolonged culture and exposure to oxidative stress, which is one of the major extrinsic factors causing SDF [Citation36]. Therefore, the fertilisation rate of IVF may be affected when there is a larger proportion of sperm with high SDF. In ICSI, a spermatozoon is injected directly into an oocyte. The better quality oocyte with less exposure to oxidative stress may better preserve its ability in repairing SDF to a certain extent [Citation37]. These factors may provide an explanation for the higher fertilisation rate in ICSI compared to IVF in the face of high SDF.
Risk of pregnancy loss
Emerging evidence in recent years has indicated an association between high SDF and an increased risk of miscarriage after ART. High SDF was associated with a significant increase in the rate of pregnancy loss after IVF and ICSI with a combined OR of 2.48 (95% CI 1.52, 4.04; P < 0.001) as reported in a systematic review [Citation29]. Another systematic review of 16 cohorts and 2969 couples confirmed a similar result and found that the risk of pregnancy loss was increased by 2.16-fold when semen specimens with high SDF were used for IVF and ICSI (95% CI 1.54, 3.03; P < 0.001) [Citation38]. Both reviews suggested that the significant associations between high SDF and miscarriage rates did not depend on the method of fertilisation used. More recently, a positive association between recurrent spontaneous abortion and high SDF has also been reported [Citation39].
A more recent systematic review and meta-analysis of 14 studies and 2756 couples indicated that elevated SDF is associated with higher miscarriage rates for men undergoing ICSI (OR 2.68, 95% CI 1.40, 5.14; P = 0.003), but not for those undergoing IVF (OR 1.84, 95% CI 0.98, 3.46) [Citation32]. The result can be explained by the different sperm selection process between the two techniques. A spermatozoon with forward motility and normal morphology is selected and injected in ICSI. However, selection with this criterion does not eliminate the chance of injecting a spermatozoon with high SDF. Indeed, morphologically normal motile sperm from infertile men have significantly higher SDF compared to those of fertile counterparts [Citation6]. The paternal effect of SDF on all stages of embryo development may explain the higher miscarriage rate in ICSI when sperm with high SDF were used [Citation40]. On the other hand, the natural selection process was not completely bypassed in the process of IVF. It is more likely that a healthier spermatozoon with less SDF would have a higher chance of fertilising the oocyte during IVF.
Although most studies indicate an association between high SDF and pregnancy loss, drawing a robust conclusion remains difficult in view of the heterogeneity of studies. The issue becomes even more complicated with the involvement of female factors. It is illustrated well that the implantation and live-birth rates during IVF/ICSI cycles in women with reduced ovarian reserve were significantly decreased when SDF exceeded 27.3%. Whilst the risk of early abortion was increased in women with normal ovarian reserve in face of high SDF; the implantation, clinical pregnancy, and live-birth rates were not affected [Citation41]. The delicate balance between SDF and oocyte repair machinery may explain the inconsistent findings from various systematic reviews and meta-analyses, as female factors were often not uniformly reported.
In summary, high SDF is strongly associated with decreased pregnancy rates in natural conception and IUI. The association between high SDF and impaired pregnancy outcomes after IVF is suggestive, but not conclusive. The implication of SDF on pregnancy outcomes becomes less clear in ICSI. However, there is fair evidence indicating high SDF may lead to increased risk to pregnancy after IVF and/or ICSI. Despite the controversy surrounding the clinical use of SDF testing, the value of SDF in predicting ART outcomes has recently been recognised by the American Society for Reproductive Medicine (ASRM) [Citation42], AUA [Citation4], and EAU [Citation5].
Implications of SDF on birth defects and the possible sequelae on genetics
The development of ICSI has revolutionised the treatment of infertility. The technique can potentially bypass even the most severe form of male factor infertility and give the infertile couple a baby. However, the safety of ART remains a concern particularly with the knowledge of SDF in the last few decades. The higher incidence of chromosomal abnormalities in ICSI candidates [Citation43] and increased rate of aneuploidy [Citation44] associated with elevated SDF validate the concern on possible genetic defects in offspring. Animal studies in mouse models have shown the negative impact of high SDF on offspring including premature ageing, aberrant growth and behaviour, and increased incidence of tumours [Citation45].
The effect of smoking and paternal age on SDF represents an indirect evidence of the impact of SDF on offspring health. Heavy smokers exhibit higher levels of SDF and oxidative adduct formation in sperm and this may explain the suggested increase in incidence of childhood cancer in the offspring of heavy smokers [Citation46]. Impaired sperm DNA integrity in ageing men has been linked with dominant genetic diseases, polygenic neurological disorders, and birth defects [Citation19,Citation47].
It is argued that there is lack of direct evidence demonstrating the deleterious effect of high SDF on the human offspring. However, the circumstantial evidence from animal studies is alarming [Citation19]. The unclear long-term consequences of transmitting defective genes, particularly in cases of extremely high SDF treated with ICSI, should not be overlooked. High-quality human study is impossible due to ethical issues. It may also require millions of ICSI children and several generations before any firm conclusion can be reached. Scepticism will persist until the question of the relationship between SDF and genetic defects is answered by longitudinal studies with sufficient samples and durations.
Treatment options for high SDF
Lack of effective treatment for high SDF has been a major obstacle in the clinical application of SDF. The situation is changing recently in view of the evolving evidence supporting different treatment strategies in alleviating SDF or selecting sperm with higher quality chromatin content for ART [Citation15]. The intake of oral antioxidants, varicocele repair, recurrent ejaculations alone or combined with sperm selection techniques, and the use of testicular sperm for ICSI, have been attempted with varying success rates. The treatment strategies for high SDF and their effects are summarised in [Citation10,Citation48–Citation54,Citation58–Citation65,Citation68,Citation70,Citation71].
Table 2 Summary of the effect on SDF using different treatment strategies.
Short abstinence
The effect of shortening the ejaculatory abstinence and repeated ejaculation as a potential means in reducing SDF in neat semen has been studied. A 22–25% reduction in the proportion of sperm with damaged DNA was achieved with ejaculatory abstinence of 1–2 days without compromising conventional semen parameters [Citation48,Citation49]. It has been shown that oxidative stress-induced SDF during epididymal transit contributes to SDF in most patients [Citation10]. This provides a possible explanation for the effect of repeated ejaculation in decreasing SDF by reducing duration of epididymal transit and possible exposure to ROS.
Oral antioxidant therapy
As oxidative stress has been investigated and found to play a detrimental role on sperm DNA integrity, the use of oral antioxidant has been studied in an attempt to reduce oxidative stress, and thus SDF. The possible beneficial effect has been suggested by studies reporting varying degrees of reduction in SDF after antioxidant therapy with different combinations of oral antioxidants [Citation50–Citation54]. The use of oral antioxidants to manage elevated SDF was studied in 38 couples with a failed ICSI attempt. An increase in clinical pregnancy rate (48.2% vs 6.9%; P < 0.05 for post- and pre-treatment with oral antioxidants) was noted after 2-months treatment with daily vitamin C and E, and a decrease in SDF was seen in 76% of men [Citation50]. A recent Cochrane review suggested a positive impact of oral antioxidants on live-birth rates in couples attending a fertility clinic (OR 4.21, 95% CI 2.08, 8.51; P < 0.001) based on a small number of studies [Citation55]. Additional studies are required involving careful selection of patients with high levels of oxidation-induced SDF and a standardised treatment regimen.
Varicocelectomy
Varicocele repair represents an effective treatment in alleviating SDF in patients with high SDF in the presence of varicocele. A systematic review involving 511 patients from 12 studies comparing men with clinical varicocele with a control group demonstrated a higher level of SDF in men with varicoceles and an improvement in SDF after varicocele repair [Citation56]. A meta-analysis also revealed a 3.37% (95% CI 2.65, 4.08; P < 0.001) reduction in SDF after varicocele repair [Citation57]. More recent studies have further shown a higher likelihood of conception after varicocelectomy associated with significant reductions in SDF. A significant decrease in SCSA SDF index from 35.2% to 30.2% (P = 0.019) was noted after varicocelectomy. Moreover, 37% of patients conceived naturally and 24% achieved pregnancy with ART after surgery. More importantly, postoperative SDF levels were significantly lower in those who achieved pregnancy whether naturally or through ART [Citation58].
Sperm selection
Sperm selection techniques including magnetic cell sorting (MACS), intracytoplasmic morphologically selected sperm injection (IMSI), and physiological ICSI with hyaluronic acid binding assay (PICSI), have brought about conflicting results [Citation60–Citation65]. A recent study with ICSI has not identified any differences in fertilisation, pregnancy, quality of embryos, implantation rates, miscarriage rates, and live-birth rates in samples prepared with or without MACS, IMSI and PICSI [Citation66]. In another report evaluating 448 ICSI cycles from couples whose male partners had high levels of SDF, the authors applied interventions to reduce SDF including IMSI and PICSI and compared outcomes with a control group of ‘no intervention’. The lowest live-birth rates of 24.2% were achieved when no intervention was adopted, whilst IMSI (28.7%) and PICSI (38.3%) resulted in modest improvements in the live-birth rate [Citation67].
Likewise, the effect of sperm preparation with density gradient centrifugation and swim-up on ART outcomes remains inconclusive; whilst some studies have found a reduction in SDF rates, others have failed to show any benefit [Citation45,Citation61,Citation65]. In addition, density gradient centrifugation has been reported to result in increased SDF, especially when higher centrifugation force and longer duration were used [Citation69].
Testicular sperm
The three- to fivefold lower SDF observed in testicular than ejaculated sperm support the use of testicular sperm for ICSI as a treatment strategy for high SDF [Citation10,Citation70,Citation71]. The reported relative reduction in SDF ranging from 66% to 80% is remarkably greater than other techniques described above (). Indeed, the beneficial effect of testicular sperm over other sperm selection techniques has been reported. A higher live-birth rate of 49.8% was reported with the use of testicular sperm and ICSI, which was significantly higher than that of IMSI (28.7%) and PICSI (38.3%) (P < 0.05) [Citation67].
Recent studies have shown promising result regarding the use of testicular sperm and ICSI in men with high SDF and oligozoospermia [Citation10,Citation72]. In one study, the authors enrolled 172 infertile men with idiopathic oligozoospermia presenting with high SDF despite oral antioxidant therapy. For the testicular sperm-ICSI group vs the ejaculated sperm-ICSI group, respectively, the live-birth rates were 46.7% and 26.4% (P = 0.007), with a RR of 1.76 (95% CI 1.15, 2.70) favouring the use of testicular sperm [Citation10]. In another study, 24 men with severe oligozoospermia who failed one or more ART cycles using ejaculated sperm with a TUNEL-positive proportion of >7% and subsequently underwent ICSI with testicular sperm were evaluated. A significantly lower TUNEL-positive rate in testicular sperm and a 50% pregnancy rate were reported with the use of testicular sperm in subsequent ICSI [Citation72]. The use of testicular sperm-ICSI in couples with previous ART failures are further supported by emerging data [Citation73,Citation74].
Clinical application of SDF tests
Whilst the importance of SDF has been increasingly supported by the literature, there seems to be insufficient evidence to endorse the routine application of SDF testing in the evaluation of infertile men [Citation4,Citation5]. Although the growing body of evidence suggests its utility in directing the management of infertile couples [Citation10,Citation67], specific indications for the test still await further research. The issue has been recently addressed by an expert panel of andrologists. The rapid advancements in SDF testing were structurally presented and specific indications for the most appropriate use of the assay were proposed based on the current best evidence [Citation75]. Potential clinical indications for SDF testing are summarised in and its rationale are presented in [Citation10,Citation25–Citation28,Citation30,Citation32,Citation33,Citation35,Citation38,Citation39,Citation41,Citation56,Citation58,Citation70,Citation72–Citation74,Citation76–Citation84].
Table 3 Clinical indications for SDF testing.
Table 4 Indications, rationale and evidence for SDF testing.
Clinical varicocele
As a substantial number of men with varicocele are able to conceive without difficulty, patient selection for varicocele repair is essential. The potential use of SDF testing in varicocele is based on the clear association between varicocele and SDF in both infertile and fertile men [Citation56]. Whilst the effect of varicocelectomy in alleviating SDF and possibly improving natural conception is supported by a few studies [Citation56,Citation58,Citation77], the relationship between SDF and varicoceles of different grades is less clear [Citation77–Citation79]. The additional information offered by SDF tests is particularly valuable when the decision to perform varicocelectomy is difficult. Therefore, SDF assays should be recommended in men with Grade 2 and 3 varicocele with normal conventional semen parameters, and in patients with Grade 1 varicocele with borderline/abnormal semen parameters ().
Unexplained infertility/recurrent pregnancy loss/IUI failure
The limitation of semen analysis is illustrated by a significant proportion of infertile couple being classified as ‘unexplained infertility’ with normal semen parameters of the male partner. The role of SDF as an additional diagnostic tool and independent predictor of male infertility status has been explored [Citation27]. It is supported by an observation of impaired sperm DNA integrity in a proportion of men with normal semen parameters and unexplained infertility [Citation80,Citation81]. Furthermore, the SDF result is highly predictive of natural pregnancy and IUI success [Citation25,Citation26]. A few studies have also reported a significantly higher SDF in couples with recurrent pregnancy loss compared to controls [Citation39,Citation82]. As a result, it is reasonable to offer SDF testing in couples with unexplained infertility or recurrent pregnancy loss for investigation of underlying aetiology. SDF tests also represent an option before initiating IUI in view of the negative implication of high SDF on IUI pregnancy rate. IVF or ICSI may be considered as the next treatment step for couples with high SDF in association with recurrent pregnancy loss or IUI failure ().
IVF and/or ICSI failure
In contrast to the modest effect of SDF on IVF outcome [Citation30,Citation35], high SDF seems to have little influence on ICSI outcomes [Citation30,Citation32]. Nevertheless, compelling evidence suggests a correlation between high SDF and pregnancy loss after both IVF and ICSI [Citation26,Citation30,Citation33,Citation38]. Amongst the treatment strategies, the use of testicular sperm represents a more promising method. In addition to the significantly lower SDF in testicular sperm [Citation10,Citation70,Citation71], higher success rates in ICSI using testicular sperm has been reported in recent studies [Citation10,Citation70,Citation72–Citation74]. Therefore, SDF testing can provide useful prognostic information on subsequent ART cycles in patients with recurrent ART failures. The use of testicular sperm in ICSI may be beneficial in this group of patients ().
Borderline abnormal semen parameters with risk factors
Exposure to various chemicals, including smoking and environmental pollutants, exerts significant impact on SDF by inducing oxidative stress [Citation83,Citation84]. SDF tests should be offered to infertile men with evidence of exposure to pollutants or found to have modifiable lifestyle risk factors (). The test result can reinforce the importance of lifestyle change, predict fertility, and monitor the response to risk factor modifications.
Conclusion
Assessment of sperm DNA damage has evolved as a valuable adjunct in the evaluation of infertile men. The importance of sperm chromatin integrity in human reproduction and its causative relationship with oxidative stress has been increasingly unveiled. The rapid development of different SDF assays has hastened advances in the field. Numerous studies have shown the relationships between SDF and reproductive outcomes in natural conception and assisted reproduction. Recent supportive evidence of effective treatment strategies in managing high SDF further consolidates the role SDF testing in the management of male factor infertility. Although there is insufficient evidence for routine SDF testing for the evaluation of infertile men, several specific clinical indications have been proposed based on the current best evidence. This represents an important step forward in promoting the wider clinical application of SDF testing and facilitating future clinical research in male infertility.
Conflict of interest
No conflicts of interest to declare.
Funding
No funding to declare.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- S.FlemingS.GreenJ.HallAnalysis and alleviation of male infertilityMicros Ann3519953739
- D.S.GuzickJ.W.OverstreetP.Factor-LitvakC.K.BrazilS.T.NakajimaC.Coutifariset alSperm morphology, motility and concentration in fertile and infertile menN Engl J Med345200113881393
- Q.V.NeriN.TanakaA.WangY.KatagiriT.TakeuchiZ.Rosenwakset alIntracytoplasmic sperm injection. Accomplishments and qualmsMinerva Ginecol562004189196
- Jarrow J, Sigman M, Kolettis PN, Lipshultz LR, McClure RD, Nangiaet AK et al. Optimal Evaluation of the Infertile Male. AUA Best Practice Statement reviewed and validity confirmed 2011. Available at: https://www.auanet.org/education/guidelines/male-infertility-d.cfm. Accessed November 2017.
- Jungwirth A, Diemer T, Kopa Z, Krausz C, Tournaye H. Male Infertility. EAU Guidelines. Available at: https://uroweb.org/guideline/male-infertility/. Accessed November 2017.
- L.SimonK.MurphyM.B.ShamsiL.LiuB.EmeryK.I.Astonet alPaternal influence of sperm DNA integrity on early embryonic developmentHum Reprod29201424022412
- L.SimonK.I.AstonB.R.EmeryJ.HotalingD.T.CarrellSperm DNA damage output parameters measured by the alkaline comet assay and their importanceAndrologia492017e1260810.1111/and.12608
- D.SakkasE.MariethozG.ManicardiD.BizzaroP.G.BianchiU.BianchiOrigin of DNA damage in ejaculated human spermatozoaRev Reprod419993137
- D.SakkasG.ManicardiP.G.BianchiD.BizzaroU.BianchiRelationship between the presence of endogenous nicks and sperm chromatin packaging in maturing and fertilizing mouse spermatozoaBiol Reprod52199511491155
- S.C.EstevesF.Sanchez-MartinP.Sanchez-MartinD.T.SchneiderJ.GosálvezComparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular spermFertil Steril104201513981405
- R.HenkelE.KierspelT.StalfC.MehnertR.MenkveldH.R.Tinneberget alEffect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patientsFertil Steril832005635642
- R.J.AitkenJ.S.ClarksonS.FishelGeneration of reactive oxygen species, lipid peroxidation and human sperm functionBiol Reprod Fertil811987459469
- R.K.SharmaA.AgarwalRole of reactive oxygen species in male infertilityUrology481996835850
- A.MajzoubS.C.EstevesJ.GosálvezA.AgarwalSpecialized sperm function tests in varicocele and the future of andrology laboratoryAsian J Androl182016205212
- A.AgarwalC.L.ChoA.MajzoubS.C.EstevesThe Society for Translational Medicine: clinical practice guidelines for sperm DNA fragmentation testing in male infertilityTransl Androl Urol6Suppl. 42017S7203310.21037/tau.2017.08.06
- R.HenkelC.F.HoogendijkP.J.BouicT.F.KrugerTUNEL assay and SCSA determine different aspects of sperm DNA damageAndrologia422010305313
- R.J.AitkenG.N.De IuliisOn the possible origins of DNA damage in human spermatozoaMol Hum Reprod162010313
- A.AgarwalC.L.ChoS.C.EstevesA.MajzoubImplication of sperm processing during assisted reproduction on sperm DNA integrityTransl Androl Urol6Suppl. 42017S5835http://10.21037/tau. 2017.04.20
- A.AgarwalC.L.ChoS.C.EstevesShould we evaluate and treat sperm DNA fragmentation?Curr Opin Obstet Gynecol282016164171
- C.L.ChoA.AgarwalA.MajzoubS.C.EstevesA single cut-off value of SDF testing does not fit allTransl Androl Urol6Suppl. 42017S501310.21037/tau.2017.08.12
- D.SakkasF.UmerD.BizzaroG.ManicardiP.G.BianchiY.Shoukiret alSperm DNA damage and altered chromatin structure: effect on fertilization and embryo developmentHum Reprod13Suppl. 419981119
- E.EvgeniG.LymberopoulosM.GazouliB.AsimakopoulosConventional semen parameters and DNA fragmentation in relation to fertility status in a Greek populationEur J Obstet Gynecol Reprod Biol18820151723
- R.A.SalehA.AgarwalD.R.NelsonE.A.NadaM.H.El-TonsyJ.G.Alvarezet alIncreased sperm nuclear DNA damage in normozoospermic infertile men: a prospective studyFertil Steril782002313318
- M.SpanòJ.P.BondeH.I.HjøllundH.A.KolstadE.CordelliG.LeterSperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study TeamFertil Steril7320004350
- G.M.Buck LouisR.SundaramE.F.SchistermanA.SweeneyC.D.LynchS.Kimet alSemen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment StudyFertil Steril1012014453462
- A.ZiniAre sperm chromatin and DNA defects relevant in the clinic?Syst Biol Reprod Med5720117885
- M.BungumP.HumaidanA.AxmonM.SpanoL.BungumJ.Erenpreisset alSperm DNA integrity assessment in prediction of assisted reproduction technology outcomeHum Reprod222007174179
- E.H.DuranM.MorshediS.TaylorS.OehningerSperm DNA quality predicts intrauterine insemination outcome: a prospective cohort studyHum Reprod17200231223128
- V.S.RilchevaN.P.AyvazovaL.O.IlievaS.P.IvanovaE.I.KonovaSperm DNA integrity test and assisted reproductive technology (Art) outcomeJ Biomed Clin Res920162129
- A.ZiniM.SigmanAre tests of sperm DNA damage clinically useful? Pros and consJ Androl302009219229
- J.A.CollinsK.T.BarnhartP.N.SchlegelDo sperm DNA integrity tests predict pregnancy with in vitro fertilization?Fertil Steril892008823831
- J.ZhaoQ.ZhangY.WangY.LiWhether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysisFertil Steril10220149981005
- L.SimonA.ZiniA.DyachenkoA.CiampiD.T.CarrellA systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcomeAsian J Androl1920178090
- M.CissenWely.MvI.ScholtenS.MansellJ.P.BruinB.W.Molet alMeasuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysisPLoS One112016e016512510.1371/journal.pone.0165125
- A.OsmanH.AlsomaitS.SeshadriT.El-ToukhyY.KhalafThe effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysisReprod Biomed Online302015120127
- J.C.DumoulinJ.A.LandA.P.Van MontfoortE.C.NelissenE.CoonenJ.G.Derhaaget alEffect of in vitro culture of human embryos on birthweight of newbornsHum Reprod252010605612
- S.E.LewisThe place of sperm DNA fragmentation testing in current day fertility managementMiddle East Fertil Soc J1820137882
- L.RobinsonI.D.GallosS.J.ConnerM.RajkhowaD.MillerS.Lewiset alThe effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysisHum Reprod27201229082917
- N.KhademA.PoorhoseyniM.JalaliA.AkbaryS.T.HeydariSperm DNA fragmentation in couples with unexplained recurrent spontaneous abortionsAndrologia462014126130
- C.AvendañoA.FranchiS.TaylorM.MorshediS.BoccaS.OehningerFragmentation of DNA in morphologically normal human spermatozoaFertil Steril91200910771084
- J.JinC.PanQ.FeiW.NiX.YangL.Zhanget alEffect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reservesFertil Steril1032015910916
- Practice Committee of the American Society for Reproductive MedicineDiagnostic evaluation of the infertile male: a committee opinionFertil Steril1032015e10825
- M.EncisoS.AlfarawatiD.WellsIncreased numbers of DNA-damaged spermatozoa in samples presenting an elevated rate of numerical chromosome abnormalitiesHum Reprod28201317071715
- R.RamasamyJ.M.ScovellJ.R.KovacP.J.CookD.J.LambL.I.LipshultzFluorescence in situ hybridization detects increased sperm anueuploidy in men with recurrent pregnancy lossFertil Steril1032015906909
- R.Fernandez-GonzalezP.N.MoreiraM.Perez-CrespoM.Sánchez-MartínM.A.RamirezE.Pericuestaet alLong-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behaviour of adult offspringBiol Reprod782008761772
- B.T.JiX.O.ShuM.S.LinetW.ZhengS.WacholderY.T.Gaoet alPaternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothersJ Natl Cancer Inst891997238244
- T.E.SchmidB.EskenaziA.BaumgartnerF.MarchettiS.YoungR.Weldonet alThe effects of male age on sperm DNA damage in healthy nonsmokersHum Reprod222007180187
- J.GosálvezM.González-MartínezC.López-FernándezJ.L.FernándezP.Sánchez-MartínShorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculateFertil Steril96201110831086
- A.AgarwalS.GuptaS.Du PlessisR.SharmaS.C.EstevesC.Cirenzaet alAbstinence time and its impact on basic and advanced semen parametersUrology942016102110
- E.GrecoS.RomanoM.IacobelliS.FerreroE.BaroniM.G.Minasiet alICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatmentHum Reprod20200525902594
- O.TuncJ.ThompsonK.TremellenImprovement in sperm DNA quality using an oral antioxidant therapyReprod Biomed Online182009761768
- Y.J.MénézoA.HazoutG.PanteixF.RobertJ.RolletP.Cohen-Bacrieet alAntioxidants to reduce sperm DNA fragmentation: an unexpected adverse effectReprod Biomed Online142007418421
- J.C.Martínez-SotoJ.C.DomingoB.CordobillaM.NicolásL.FernándezP.Alberoet alDietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decrease sperm DNA fragmentationSyst Biol Reprod Med622016387389
- C.AbadM.J.AmengualJ.GosálvezK.CowardN.HannaouiJ.Benetet alEffects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNAAndrologia452013211216
- M.G.ShowellR.Mackenzie-ProctorJ.BrownA.YazdaniM.T.StankiewiczR.J.HartAntioxidants for male subfertilityCochrane Database Syst Rev122014 CD007411
- A.ZiniG.DohleAre varicoceles associated with increased deoxyribonucleic acid fragmentation?Fertil Steril96201112831287
- Y.J.WangR.Q.ZhangY.J.LinR.G.ZhangW.L.ZhangRelationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysisReprod Biomed Online252012307314
- M.SmitJ.C.RomijnM.F.WildhagenJ.L.VeldhovenR.F.WeberG.R.DohleDecreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rateJ Urol1832010270274
- A.ZiniR.AzharA.BaazeemM.S.GabrielEffect of microsurgical varicocelectomy on human sperm chromatin and DNA integrity: a prospective trialInt J Androl3420111419
- T.H.LeeC.H.LiuY.T.ShihH.M.TsaoC.C.HuangH.H.Chenet alMagnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertilityHum Reprod252010839846
- M.NadaliniN.TarozziM.Di SantoA.BoriniAnnexin V magnetic-activated cell sorting versus swim-up for the selection of human sperm in ART: is the new approach better than the traditional one?J Assist Reprod Genet31201410451051
- I.HammoudF.BoitrelleF.FerfouriF.VialardM.BergereB.Waineret alSelection of normal spermatozoa with a vacuole-free head (x6300) improves selection of spermatozoa with intact DNA in patients with high sperm DNA fragmentation ratesAndrologia452013163170
- R.MaettnerK.SterzikV.IsachenkoE.StrehlerG.RahimiJ.L.Alabartet alQuality of human spermatozoa: relationship between high magnification sperm morphology and DNA integrityAndrologia462014547555
- L.ParmegianiG.E.CognigniS.BernardiE.TroiloW.CiampagliaM.Filicori‘Physiologic ICSI’, Hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo qualityFertil Steril932010598604
- L.Rashiki GhalenoM.Rezazadeh ValojerdiM.ChehraziF.Sahraneshin SamaniYazdi R.SalmanHyaluronic acid binding assay is highly sensitive to select human spermatozoa with good progressive motility, morphology, and nuclear maturityGynecol Obstet Invest812016244250
- K.L.RappaH.F.RodriguezG.C.HakkarainenR.M.AnchanG.L.MutterW.AsgharSperm processing for advanced reproductive technologies: where are we today?Biotechnol Adv342016578587
- C.K.BradleyS.J.McArthurA.J.GeeK.A.WeissU.SchmidtL.ToogoodIntervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysisAndrology42016903910
- X.XueW.S.WangJ.Z.ShiS.L.ZhangW.Q.ZhaoW.H.Shiet alEfficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patientsJ Assist Reprod Genet31201411611166
- A.ZiniR.K.NamV.MakD.PhangK.JarviInfluence of initial semen quality on the integrity of human sperm DNA following semen processingFertil Steril742000824827
- E.GrecoF.ScarselliM.IacobelliL.RienziF.UbaldiS.Ferreroet alEfficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoaHum Reprod202005226230
- S.I.MoskovtsevK.JarviJ.B.MullenK.I.CadeskyT.HannamK.C.LoTesticular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatmentFertil Steril93201011421146
- A.MehtaA.BolyakovP.N.SchlegelD.A.PaduchHigher pregnancy rates using testicular sperm in men with severe oligospermiaFertil Steril104201513831387
- E.G.PabuccuG.S.CaglarS.TangalA.H.HalilogluR.PabuccuTesticular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failuresAndrologia492017e1260910.1111/and.12609
- M.ArafaH.ElbardisiA.AlMalkiH.BurjaqA.MajzoubS.AlSaidet alICSI outcome in patients with high DNA fragmentation: testicular vs ejaculated spermAndrologia201710.1111/and.12835 [Epub ahead of print]
- A.AgarwalA.MajzoubS.C.EstevesE.KoR.RamasamyA.ZiniClinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenariosTransl Androl Urol52016935950
- S.C.EstevesJ.GosálvezC.López-FernándezR.Núñez-CalongeP.CaballeroA.Agarwalet alDiagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertilityInt Urol Nephrol47201514711477
- K.NiK.StegerH.YangH.WangK.HuB.ChenSperm protamine mRNA ratio and DNA fragmentation index represent reliable clinical biomarkers for men with varicocele after microsurgical varicocele ligationJ Urol1922014170176
- A.SadekA.S.AlmohamdyA.ZakiM.ArefS.M.IbrahimT.MostafaSperm chromatin condensation in infertile men with varicocele before and after surgical repairFertil Steril95201117051708
- S.V.Krishna ReddyA.B.ShaikS.SailajaM.VenkataramanaiahOutcome of varicocelectomy with different degrees of clinical varicocele in infertile maleAdv Androl201510.1155/2015/432950
- R.A.SalehA.AgarwalE.A.NadaM.H.El-TonsyR.K.SharmaA.Meyeret alNegative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertilityFertil Steril79Suppl. 3200315971605
- K.OleszczukL.AugustinessonN.BayatA.GiwercmanM.BungumPrevalence of high DNA fragmentation index in male partners of unexplained infertile couplesAndrology12013357360
- H.B.FordD.J.SchustRecurrent pregnancy loss: etiology, diagnosis, and therapyRev Obstet Gynecol220097683
- F.YangL.LiJ.P.ChenX.Q.LiuC.L.ZhongY.Yanget alCouple’s infertility in relation to male smoking in a Chinese rural areaAsian J Androl192017311315
- G.U.WijesekaraD.M.FernandoS.WijerathnaN.BandaraEnvironmental and occupational exposures as a cause of male infertilityCeylon Med J6020155256