Abstract
Despite being first described two thousand years ago, the varicocele remains a controversial multifaceted disease process with numerous biological consequences including infertility, hypogonadism, and chronic orchidalgia. The underlying mechanisms remain poorly understood and likely include hypoxia, oxidative stress, hyperthermia, anatomical aberrations, and genetics as primary components. Despite a high prevalence amongst asymptomatic fertile men, varicoceles paradoxically also represent the most common correctable cause for male infertility. In this systematic review we discuss the rich historical aspects of the varicocele and the contemporary data regarding its clinical manifestations. We performed a systematic literature review with the goal of comparing outcomes and complication rates of each of the major surgical approaches as they relate to infertility and pain. We performed a Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)-compliant systematic literature review for manuscripts focused on varicocele and its biological consequences. We identified 112 studies suitable for qualitative analysis and included 56 of these for quantitative analysis, with an emphasis on infertility and chronic pain outcomes. Taken together, the clinical work to date suggests that the highest fertility rates and the lowest complication rates are associated with the microsurgical subinguinal surgical approach to varicocelectomy. In all, 26–40% of patients undergoing varicocelectomy will successfully achieve short-term spontaneous pregnancy, and up to 90% of all patients undergoing varicocelectomy for pain will have improvement and/or resolution of their symptoms. Taken together, the data support an ongoing role for varicocelectomy in both of these clinical arenas.
Introduction
Overview and epidemiology
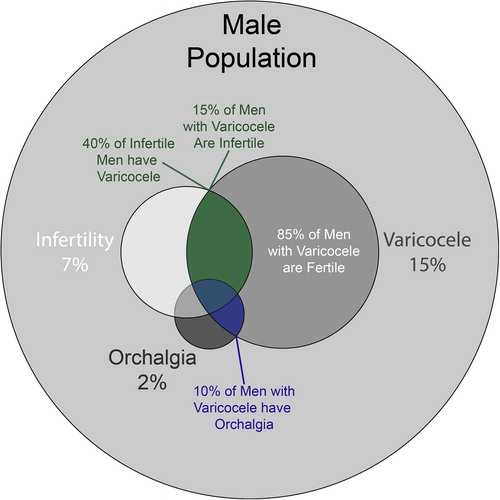
A varicocele is defined as a dilated pampiniform plexus, the network of small veins responsible for venous drainage from the testicle and deep tissues of the hemiscrotum. This plexus is contiguous with the ipsilateral gonadal vein, which drains into the renal vein on the left and directly into the inferior vena cava on the right. As a result, the left renal vein is typically 8–10 cm longer and has a higher hydrostatic pressure; this explains the discrepancy in incidence between the left side (which accounts for 90% of all varicoceles) and the right side, which if tense and unilateral may be concerning for malignancy [Citation1]. Epidemiologically, varicoceles are common and occur in 15% of the general male population ( ) [Citation2,Citation3]. Varicoceles typically develop during puberty. A large population-based study showed a prevalence of 0.92% in boys aged between 2 and 10 years and a dramatic rise to 11% in boys aged 11–19 years [Citation4]. Men presenting with infertility have an even higher prevalence, ranging from 35% for men presenting with primary infertility [Citation5] to 45–81% for those presenting with secondary infertility [Citation5,Citation6].
Historical perspective
The initial description of the varicocele was published nearly 2000 years ago by Celsus, who stated that ‘The veins are swollen and twisted over the testicle, which becomes smaller than its fellow, in as much as its nutrition has become defective’ [Citation7]. Ambroise Paré in 1550 described ‘a dilatation of a vein, filled with melancholy blood, and often growing in men of melancholy temper’ [Citation2]. Nearly 300 years later, the French surgeon Delpech was murdered by a disgruntled patient who underwent bilateral varicocele repair and developed testicular atrophy [Citation8]. It would take yet another 100 years before the varicocele was recognised and treated as a potential source for infertility, when Tulloch, a Scottish surgeon, published his first series of 30 patients in 1955 [Citation9]. In his manuscript, he describes an azoospermic gentleman with bilateral varicoceles who underwent varicocelectomy and postoperatively was noted to have a sperm count of 27 million and went on to father a child. Since then, hundreds of manuscripts have been published describing various surgical approaches, biological effects, and indications for repair for this multifaceted disease process. In this review article, we summarise this knowledge base via a systematic review and identify the historical and contemporary aspects of the diagnosis and management of varicoceles. We also report a quantitative summary of the outcomes to date in the modern era for both infertility and pain outcomes stratified by surgical approach.
Methods
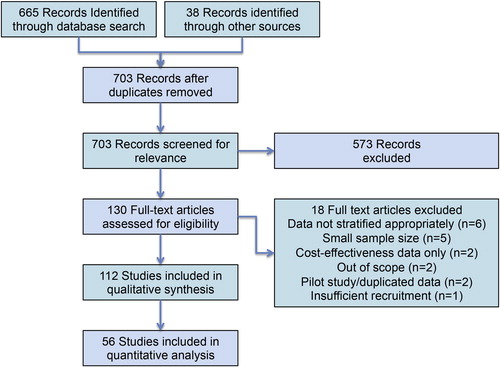
This article presents a systematic review of previously published studies; therefore, ethical approval and written informed consent from patients was not required. This research was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. We performed a comprehensive literature review for the years 1995–2017 via PubMed and Cochrane Library. The review was consistent with the PRISMA criteria. The initial search was conducted with the following search string: (‘varicocele’[Title] OR ‘varicoceles’[Title] OR ‘varicocelectomy’[Title]) AND (‘infertility’[Title/Abstract] OR ‘pain’[Title/Abstract]) AND (‘1995’[Date – Publication]: ‘2017’[Date – Publication]). This search identified 665 records; an additional 38 reports were identified via searching the references of relevant manuscripts and recent published abstracts and considered for inclusion. Exclusion criteria included the following; lack of clinical applicability, manuscripts in languages other than English, retracted articles, and duplicated articles. We further excluded manuscripts from the quantitative analysis based upon a number of other criteria ().
Results
Following the literature search and application of exclusion criteria, 112 studies were included in the final qualitative synthesis (). Of these, 56 were used to perform a quantitative analysis with a focus on postoperative outcomes stratified by indication and by surgical approach.
Biological consequences
Pain
Varicoceles are typically asymptomatic, but ∼10% will present with a chief complaint of scrotal pain [Citation10]. The pain is typically a heavy full pain that is worse with standing or heavy activity. The pain can be subacute or chronic and can be bilateral or unilateral. Regardless of the specific presentation, scrotal pain must be adequately investigated to exclude acute pathology and then subsequently managed appropriately.
Testicular atrophy
Varicocele-associated testicular atrophy was first described in detail by Lipshultz and Corriere [Citation11] in 1977, and subsequently confirmed with multiple studies with a prevalence of ∼10% [Citation4]. Atrophy is commonly defined as a >10% decrease in volume compared to the contralateral testis, or in the case of bilateral insults, a decrease in size below the expected testicular volume based upon age and Tanner stage.
Infertility
Nearly 10% of men visit a medical professional for infertility evaluation during their reproductive years, and the most common identified correctable cause of infertility within this population is the presence of a varicocele [Citation12,Citation13]. Infertility is perhaps the best studied, most complex, and enigmatically still one of the most controversial aspects of varicoceles and urology in general. From a population perspective, 16% of men with confirmed fertility (i.e. fathered at least one child) had a varicocele at the time of vasectomy [Citation14]. Others have shown that most men with a varicocele have normal semen parameters [Citation3], suggesting a complex interplay between infertility and the presence of a varicocele. Despite these statistics, the link between infertility and varicoceles has been well documented for >50 years and a great deal of correlative data have been published. Importantly, however, subclinical varicoceles (those impalpable on examination and identified only radiologically) do not appear to have clinically significant effects on semen parameters. A well-executed prospective randomised trial of 68 patients with subclinical left varicoceles showed no improvement in either semen parameters or paternity rates with ligation [Citation15]. Whilst the study was probably underpowered to detect subtle changes, the data currently support the notion that subclinical varicoceles should not be treated.
Hormonal effects
The hormonal effects of the varicocele were first postulated >150 years ago, when Curling noted a ‘decrease in the secreting powers of the gland’ [Citation8]. Whilst much of the negative effects on spermatogenesis can be attributed to Sertoli cell and germ cell insults, the hormonal effects of varicoceles suggest a pantesticular effect that includes Leydig cells and impaired testosterone production. Interestingly, the historical data have produced conflicting results on whether the presence of a varicocele is associated with hypogonadism. However, a recent meta-analysis by Li et al. [Citation16] showed baseline diminished testosterone production in men with varicoceles and subsequent improvement with varicocelectomy. In contrast, one of the largest series to date failed to identify a correlation between varicocele of any grade and either total or free testosterone levels in healthy young males [Citation3]. However, this study did identify clear differences in nearly every other serum hormonal parameter studied, including FSH, LH, and inhibin B. Taken together, the data suggest several hypotheses. First, perhaps the changes in testosterone production with a varicocele occur gradually in the setting of ongoing chronic Leydig cell stress and only manifest as hypogonadism in older men beyond their prime reproductive years. Alternatively, perhaps a subset of patients with a varicocele may be predisposed to exaggerated effects on hormonal production; it is likely that, if true, this population could benefit most from varicocelectomy (at least from an endocrine perspective).
Mechanisms of testicular dysfunction
Anatomical and genetic components
Historically, the testicular dysfunction associated with a varicocele was thought to be predominately structural in nature. Early anatomical studies showed that gonadal veins associated with a varicocele lacked anti-reflux valves responsible for preventing retrograde flow and reflux. Work from the radiology literature has shown that in healthy men, 40% had incompetent valves on the left, compared to 23% on the right [Citation17]. These findings contributed to the notion that elevated venous pressure plays a key role in the pathophysiology of the varicocele. These anatomical findings may also correlate to a genetic component. A genetic study in 2005 examined first-degree relatives of men with a varicocele and found that 57% also had a clinically palpable varicocele, as compared to 7% in the control group [Citation18]. Taken together, these data suggest a genetic and/or anatomical link underlying at least some of the biological consequences of having a varicocele.
Hyperthermia
A critical component of spermatogenesis is the maintenance of the proper testicular temperature ∼2 °C below body temperature. Disruption of this relatively cool environment by external influences, e.g. hot tubs, has been proposed to impair spermatogenesis and contribute to infertility. The mechanism by which the male genitalia control this delicate temperature balance was first proposed by Dahl and Herrick [Citation19] in 1959. Briefly, this phenomenon relies on a counter-current heat exchange system via closely approximated arteries and veins, which allows heat from the arterial flow to escape and warm the venous flow and keep the scrotum cooler. Work by Zorgniotti and Macleod [Citation20] showed a 0.6 °C difference in oligospermic patients with varicoceles as compared to controls. Subsequent studies with refined techniques and intratesticular temperature measurements showed an even larger difference reaching 2–3 °C in magnitude [Citation21]. When considering rapid heat exchange that occurs across the scrotal septum, this mechanism is also concordant with the notion that a unilateral pathology results in bilateral dysfunction. The precise molecular pathway by which temperature so profoundly affects spermatogenesis is not entirely known, but evolving research suggests that heat shock proteins (HSPs) may play a key role in this phenomenon. These proteins are highly conserved proteins that assist in protein folding and stress response. HSPA2, a chaperone for cytoplasmic and mitochondrial proteins, has been shown to be critical in the prevention of apoptosis, and men with oligospermia and varicoceles have been shown to have significantly decreased expression relative to men with varicocele and normal sperm concentrations [Citation22]. Hosseinifar et al. [Citation23] used a proteomics approach to compare pre- and post-varicocelectomy protein expression in 20 men desiring surgical management for a grade 3 varicocele. Three proteins were differentially downregulated in the presence of a varicocele, and one of these proteins was HSPA5. In summary, these studies suggest that hyperthermia induces a stress-state within the testicle and alters gene expression, predisposing cells to apoptosis.
Hypoxia
Despite being a relatively well-vascularised organ, the testicle could conceivably become hypoxic if the venous backpressure exceeds the arterial inflow pressure, limiting flow and oxygenation. Unlike the acute ischaemia accompanying testicular torsion, hypoxia from a varicocele is a chronic process and results in compensatory changes within the testicle. The best-studied gene within this domain is hypoxia-inducible factor-1α (HIF1A), a transcription factor involved in erythropoiesis, angiogenesis, and mitochondrial respiration. Work in the mid-2000s by several groups showed that HIF1A and vascular endothelial growth factor (VEGF, another angiogenic protein) are both significantly upregulated in the presence of a varicocele [Citation24,Citation25].
Oxidative stress and reactive oxygen species (ROS)
It is now well recognised that oxidative stress plays a significant role in spermatogenesis and semen analysis parameters. The first implication of ROS in the setting of a varicocele was published in 1999, when Hendin et al. [Citation26] reported elevated ROS in both fertile and infertile men with varicocele. This work was further validated in a subsequent meta-analysis [Citation27]. The mechanism underlying this finding can be at least partially attributed to a disequilibrium between ROS and ROS-scavenging mechanisms within the walls of varicose veins [Citation28] and varicoceles in particular [Citation29]. The grade of varicocele appears to linearly correlate with the degree of ROS production, as well as the impairment on sperm quality, but this does not necessarily correlate with testicular size [Citation30]. It appears that varicocelectomy has a significant beneficial effect on improving the oxidative environment by decreasing ROS and allowing antioxidant levels to increase [Citation31]. Further work has shown that these parameters normalise to levels comparable with controls [Citation32], suggesting that the oxidative stress aspect of varicoceles can be reversible with treatment.
Other hypothesised mechanisms
Given the numerous conflicting reports and lack of a unified hypothesis to explain the pathophysiology of the varicocele, several other hypotheses have been put forth. These include the compromise of the blood–testis barrier [Citation33], adrenal metabolite reflux [Citation34], and impaired spermatogenesis due to hormonal imbalance. Despite these preliminary studies, it remains to be seen whether these less-understood phenomena play a significant role in testicular dysfunction with a varicocele.
Clinical presentation
Varicoceles are commonly identified in one of three presentations. First, young males presenting for routine examination are noted to have an asymptomatic varicocele on physical examination. Second, men of reproductive age note difficulty with conception and present to their fertility specialist with a history of primary infertility and a clinically relevant varicocele is noted. Finally, middle-aged men occasionally present with chronic orchidalgia refractory to conservative measures and are diagnosed with a varicocele (which may or may not be related to their symptoms). In any of these cases, further clinical investigation is warranted before pursuing surveillance or treatment.
Diagnosis
History and physical examination
The most important diagnostic consideration in the identification and evaluation of a varicocele is a careful history and physical examination. Whilst more advanced diagnostic methods, e.g. imaging, can often identify subclinical varicoceles, much of the management decisions hinge upon whether the varicocele is palpable by a careful examination by an experienced clinician. Men should ideally be examined in a warm environment, and some clinicians even advocate for extrinsic heat via heating pads to maximally relax the scrotum. The patient should be examined in both the upright and recumbent positions, and any varicocele that does not reduce in the supine position may be concerning for precipitating pathology, such as retroperitoneal neoplasms. The addition of a Valsalva manoeuvre to the examination allows for exaggerated venous congestion and is necessary for accurate grading. Orchidometry may also play a role in objectively assessing for testicular volume and may be nearly as accurate as ultrasonography (US) in this regard according to a recent study, which noted a 97.8% concordance to within 10% of measured volume on orchidometry vs US [Citation35].
Imaging
Unlike many areas of urology, imaging does not play a primary role in the diagnosis and management of varicoceles and is reserved only for specific clinical scenarios. US has become the most widely used imaging method for the diagnosis of testicular pathologies. Colour Doppler US in particular offers good spatial resolution and identification of venous congestion, and studies have shown good concordance with physical examination in this regard [Citation36]. However, US is not indicated for routine varicocele diagnosis, and physical examination remains the ‘gold standard’ and should be the primary factor driving management decisions. However, US can be useful in particular clinical scenarios where the physical examination is limited (e.g. challenging body habitus or thick scrotal skin). The primary concern with US is the over diagnosis of subclinical varicoceles, which have been shown to have little or no effect on semen quality [Citation15].
Prior to the widespread use of US, venography was widely used for both diagnostic and therapeutic purposes. The technique is performed using the Seldinger technique to place a catheter from the internal jugular or femoral vein into the testicular vein and injecting contrast to assess for reflux. If present, the varicocele can then be simultaneously treated using embolisation. Whilst the test is widely considered to be the most sensitive test to diagnose a varicocele, it suffers from poor specificity, high variability between providers, and invasiveness, and should be only offered in select circumstances.
Grading
Numerous grading schemas have been developed for the quantitative grading of varicoceles [Citation36]. The most common clinical grading scale was developed by Dubin and Amelar [Citation37] ( [Citation37,Citation38]) and stratifies the lesion based upon whether the varicocele is palpable or visible with or without a Valsalva manoeuvre. Radiographic grades via US are most commonly assigned within the Sarteschi classification [Citation38] (), although Chiou et al. [Citation36] have proposed a modified scale designed to more closely approximate clinical grading and physical examination findings.
Table 1 Clinical and sonographic varicocele classification schemes.
Indications for repair
Infertility
According to the AUA Best Practice Statement on Varicocele and Infertility, treatment should only be offered for men with palpable varicoceles and abnormal semen parameters [Citation39]. If the patient is actively attempting to conceive, varicocelectomy should only be offered if the partner has normal or potentially correctable infertility conditions (or he currently has no partner and wishes fertility in the future).
Adolescents
The adolescent male population with a clinical varicocele remains controversial with regard to management approaches. Clearly not all young men with a varicocele require treatment, and yet the development of testicular atrophy undoubtedly contributes to the growing population of men with infertility. The data to date are fairly convincing that testicular volume in these young men improves after repair [Citation40,Citation41], suggesting that early intervention may be beneficial from a patient and an epidemiological standpoint. As a result, current guidelines suggest that young men with the presence of a varicocele should be monitored carefully and should be offered treatment only if there is objective evidence of reduced ipsilateral testicular size or abnormal semen parameters. Despite this recommendation, there are still conflicting data on whether adolescent repair confers a fertility benefit. On one hand, Bogaert et al. [Citation42] retrospectively saw no difference in fertility rates between patients who underwent repair as an adolescent and those who did not. On the other hand, Çayan et al. [Citation43] saw a dramatic increase in fertility rates in men who underwent adolescent varicocelectomy. Given these contradictory findings, a prospective randomised trial will likely be necessary to address this question.
Pain
Chronic orchidalgia represents a difficult diagnostic and therapeutic dilemma, and the presence of a varicocele can confound this to a degree. Whilst some men who ultimately undergo varicocelectomy will have improvement or resolution of their symptoms [Citation10], not all men benefit from the intervention. Based upon this, chronic pain can be an indication for repair, but only after conservative measures are exhausted and a thorough discussion has occurred with the patient about the risks and benefits.
Bilateral varicocele
Up to 50% of men with unilateral varicocele will ultimately be diagnosed with bilateral disease. Scherr and Goldstein [Citation113] studied this population by performing either unilateral or bilateral repairs in men with a grade II or III left varicocele and a grade I or higher right varicocele. They found that patients undergoing bilateral repair had significant improvements in semen analysis parameters, suggesting that if unilateral repair is indicated otherwise, patients with bilateral clinical disease should be offered bilateral repair from an infertility standpoint.
Surgical treatment
Historical
Surgical management for varicoceles was first described in 25–35 AD by Celsus, who applied crude suture ligatures and thin cauterising irons to manage dilated scrotal veins. Numerous other surgeons subsequently described techniques involving venous ligature, cauterisation, and even partial scrotectomy for increased ‘inner support’ of the testicle [Citation8]. The first series in the modern era was published by Barwell [Citation44] in 1885, who reported in The Lancet his series of 100 cases treated with a wire loop placed around the dilated veins and the subsequent improvement in testicular size. Numerous other surgical approaches have been described, each with benefits and drawbacks.
Open surgical approaches
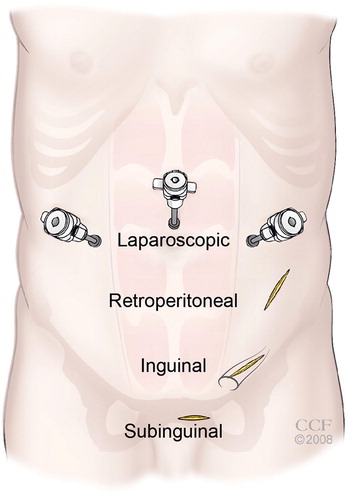
A variety of open surgical techniques have been described ( ). Due to a high rate of testicular artery damage and/or hydrocoele formation, the scrotal approach is a historical operation that is rarely employed in the modern era and has been replaced by safer and more reliable approaches. The retroperitoneal high ligation technique, known as the Palomo approach [Citation45], utilises a horizontal incision medial to the anterior superior iliac spine. This approach enables identification of the internal spermatic vein before extensive branching, which theoretically could reduce the recurrence rate. This approach can be performed with or without arterial ligation. Disadvantages include: increased pain due to the additional necessary tissue dissection, higher hydrocoele rates, and an inability to identify the external spermatic veins resulting in increased recurrences. The inguinal (also known as Ivanissevich [Citation46]) approach is a traditional surgical dissection in familiar anatomy, but the inguinal canal dissection requires fascial incision and increases the risk of pain and hernia formation, as well as inadvertent damage to the ilioinguinal nerve. More recently, the subinguinal (Goldstein) approach has gained popularity as a safe and effective operation. Benefits include a shorter recovery and less pain than the inguinal approach (probably due to the lack of fascial violation). Disadvantages include a longer operative time [Citation47], presumably due to the slightly less intuitive anatomy.
Laparoscopic repair
The laparoscopic intraperitoneal approach, introduced by Sanchez-de-Badajoz et al. [Citation48] in 1990 utilises a transperitoneal intra-abdominal approach, which offers several advantages including increased efficiency for bilateral surgery and relatively short operating times. The approach involves placement of laparoscopic ports in the abdomen, identifying the inguinal ring and the spermatic cord contents, and selectively ligating the gonadal veins, whilst leaving the arterial blood supply intact. However, this approach is an intra-abdominal procedure and carries a small added risk for complications, e.g. visceral injury from trocar placement.
Microsurgical technique
The microscopic approach, first published by Marmar et al. [Citation49] in 1985 and further refined by Goldstein et al. [Citation50], involves a subinguinal approach to the cord and offers a high success rate and minimal postoperative pain at the expense of requiring an operating microscope and comfort with microsurgical techniques. Due to the anatomy of this venous plexus, the average number of vessels that must be controlled at this level is higher as compared to the inguinal canal.
Endovascular approaches
In stark contrast to surgical management, the Tauber approach [Citation51] utilises antegrade injection of a sclerosing agent directly into the pampiniform plexus via a small incision. This technique is straightforward, relatively painless, and carries relatively minimal risk. The retrograde sclerotherapy or coil embolisation approach avoids surgical incisions entirely and instead relies on retrograde cannulation of the testicular vein and injection of the appropriate agent to cause venous obstruction. Disadvantages to these techniques include a relatively high rate of recurrence of up to 15% [Citation52] and the necessity for experienced interventional radiology or urology providers.
Surgical complications
Regardless of the approach, the complications after surgical or percutaneous intervention are relatively similar. Postoperative pain and haematoma can occur to varying degrees but typically improve or resolve over time. Hydrocoele formation, typically attributed to the ligation of lymphatics, can be problematic and occurs with varying frequency depending on the specific surgical approach ( [Citation70Citation[71]Citation[72]Citation[73]Citation[74]Citation[75]Citation[76]Citation[77]Citation[78]Citation[79]Citation[80]Citation[81]Citation[82]Citation[83]Citation[84]Citation[85]Citation[86]Citation[87]Citation[88]Citation[89]Citation[90]Citation[91]Citation[92]Citation[93]Citation[94]Citation[95]Citation[96]Citation[97]Citation[98]Citation[99]Citation[100]–Citation101]). Likewise, the recurrence of the varicocele can occur with any surgical or percutaneous intervention (). In summarising the collective surgical literature to date, the risk of hydrocoele formation appears to be lowest with the microsurgical subinguinal approach (0.6%). This is followed by the open inguinal (5.3%), laparoscopic (6.7%), and finally is highest with the retroperitoneal (7.5%) approach (). Similarly, the microscopic subinguinal repair is associated with the lowest rate of recurrence and/or technical failure at 1.2% of cases, whereas the highest failure rate is associated with retroperitoneal repair (12.6%). Taken together, these data suggest that the microsurgical subinguinal technique carries the lowest risk of hydrocoele and varicocele recurrence and may represent the optimal surgical strategy in experienced hands.
Table 2 Varicocelectomy infertility and complications outcomes by surgical approach.
Outcomes
Fertility outcomes
Numerous retrospective and prospective studies have now been completed regarding the effects of varicocelectomy on semen quality and fertility. The first trial that attempted to definitively answer this question was a randomised controlled trial published by Nieschlag et al. [Citation53] in 1998. This study was plagued by poor patient accrual and completion rates and by differences in baseline epidemiological characteristics (including the age of the female partner) between groups. The study found no difference in spontaneous pregnancy rates between observation and intervention groups. A series of Cochrane Review manuscripts were then published based upon this and other studies, and failed to show a significant difference in pregnancy rates for patients undergoing varicocelectomy [Citation54,Citation55]. Notably, however, these studies included men with subclinical varicoceles, which have been shown to have no bearing on fertility. Subsequently, several other meta-analyses that focused on the impact on varicocelectomy on semen parameters in men with clinically relevant varicoceles [Citation56Citation[57]–Citation58] uniformly reported improved sperm concentration, motility, and morphology compared to observation. Abdel-Meguid et al. [Citation59] then reported data from a randomised controlled trial involving 145 infertile participants with palpable varicoceles and abnormal semen analysis randomised to observation or varicocelectomy. The study followed up these men for 12 months and noted a statistically significant increase in spontaneous pregnancy rates (the primary endpoint) in the varicocelectomy group with an odds ratio of 3.04. Motivated by these results, a follow up Cochrane review in 2012 [Citation60] analysed data from nearly 900 men from 10 studies and showed an improvement in spontaneous pregnancy rates, which was even more robust if subclinical varicoceles were removed in a subgroup analysis with a number needed to treat of 17. The study noted that the data interpretation was still limited by high study heterogeneity and low quality evidence. Taken together, these data increasingly suggest a reproducible positive benefit for varicocelectomy for infertile men, although the benefit remains less substantial than what was initially expected. In comparing the spontaneous pregnancy rates associated with varicocele repair, the microscopic subinguinal approach appears to convey the greatest positive benefit, with 41% of postoperative patients achieving spontaneous pregnancy ().
Pain
Varicocelectomy for chronic orchidalgia has been studied less intensely than for infertility indications, but nevertheless a growing number of studies are beginning to shed light on this indication. The first large study was completed and published in 2000 and enrolled 119 men who underwent varicocelectomy for pain [Citation61]. The study reported an 88% complete response rate and a 5% partial response. Several other more recent smaller studies appear to corroborate the relatively high success rate of these early studies [Citation62Citation[63]–Citation64]. A recent review paper has elegantly summarised these results, and taken together the success rate for varicocelectomy in patients with orchidalgia approaches 90% [Citation10]. In stratifying these collective data by surgical approach, the highest pain-free rates to date have been reported with the subinguinal microscopic approach ( ). The inguinal and retroperitoneal approaches appear to be slightly less effective, with 75% and 76% of men reporting improvement in pain, respectively ( [Citation61Citation[62]Citation[63]–Citation64,Citation102Citation[102]Citation[103]Citation[104]Citation[105]Citation[106]Citation[107]Citation[108]Citation[109]Citation[110]Citation[111]–Citation112]). In summary, the data seem to suggest a robust improvement in orchidalgia in most carefully selected patients who have previously failed conservative management.
Table 3 Varicocelectomy for pain and aggregate outcomes by surgical approach.
Hormonal effects
Perhaps the least studied indication for varicocelectomy is hypogonadism. Nevertheless several studies [Citation65Citation[66]Citation[67]Citation[68]–Citation69] have shown robust increases in serum testosterone, particularly in hypogonadal men, after repair. Whilst further prospective randomised controlled studies are necessary, these data suggest that perhaps hypogonadism may represent another indication for varicocelectomy in the properly selected patient.
Conclusions
From its original description 2 millennia ago, our understanding of varicoceles has transformed from a poorly defined oddity to a well-characterised condition with increasingly clear indications for conservative and surgical management. Despite the multitude of studies and data describing the various management options and the impact on fertility, pain, and hypogonadism, controversy still exists in several key areas. The precise mechanism (or more accurately mechanisms) contributing to varicocele-induced infertility and their relative contributions remains elusive. The role of elective varicocelectomy in the hypogonadal man also remains to be fully elucidated and validated. Finally, one of the greatest challenges remaining from an infertility standpoint centres on the fact that up to 25% of men with grade 2 and 17% of men with grade 3 varicocele have high semen quality [Citation3], and simultaneously only a minority of men who undergo a varicocelectomy will subsequently contribute to a spontaneous pregnancy. Moving forward, it will be important to better understand this heterogeneity and better stratify men into those likely to benefit from intervention, and those unlikely to do so.
Conflict of interest
None.
Source of funding
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- D.G.KaufmanH.M.NaglerThe varicocele: concepts of pathophysiology – present and futureWorld J Urol41986889110.1007/BF00326400
- D.C.SaypolVaricoceleJ Androl21981617110.1002/j.1939-4640.1981.tb00597.x
- J.DamsgaardU.N.JoensenE.CarlsenJ.ErenpreissM.B.JensenV.Matuleviciuset alVaricocele is associated with impaired semen quality and reproductive hormone levels: a study of 7035 healthy young men from six European countriesEur Urol7020161019102910.1016/j.eururo.2016.06.044
- E.AkbayS.CayanE.DorukM.N.DuceM.BozluThe prevalence of varicocele and varicocele-related testicular atrophy in Turkish children and adolescentsBJU Int862000490493
- J.I.GorelickM.GoldsteinLoss of fertility in men with varicoceleFertil Steril591993613616
- J.P.JarowM.CoburnM.SigmanIncidence of varicoceles in men with primary and secondary infertilityUrology471996737610.1016/S0090-4295(99)80385-9
- P.MassonR.E.BranniganThe varicoceleUrol Clin North Am41201412914410.1016/j.ucl.2013.08.001
- H.D.NöskeW.WeidnerVaricocele – a historical perspectiveWorld J Urol171999151157
- W.S.TullochVaricocele in subfertilityBr Med J21955356358
- R.C.OwenB.J.McCormickB.D.FiglerR.M.CowardA review of varicocele repair for painTransl Androl Urol6Suppl 12017S20S2910.21037/tau.2017.03.36
- L.I.LipshultzJ.N.CorriereProgressive testicular atrophy in the varicocele patientJ Urol1171977175176
- P.McGarryK.AlrabeeahK.JarviA.ZiniInfertility is varicocelectomy beneficial in men previously deemed subfertile but with normal semen parameters based on the new guidelines? A retrospective study.Urology85201535736210.1016/j.urology.2014.10.031
- L.DubinR.D.AmelarEtiologic factors in 1294 consecutive cases of male infertilityFertil Steril221971469474
- M.P.de CastroD.A.MastroroccoReproductive history and semen analysis in prevasectomy fertile men with and without varicoceleJ Androl519841720
- M.GrassoC.LaniaM.CastelliL.GalliF.FranzosoP.RigattiLow-grade left varicocele in patients over 30 years old:the effect of spermatic vein ligation on fertilityBJU Int852000305307
- F.LiH.YueK.YamaguchiK.OkadaK.MatsushitaM.Andoet alEffect of surgical repair on testosterone production in infertile men with varicocele: A meta-analysisInt J Urol19201114915410.1111/j.1442-2042.2011.02890.x
- N.E.AhlbergO.BartleyRight and left gonadal veins: an anatomical and statistical studyActa Radiol Diagn (Stockh)41966593601
- J.D.RamanK.WalmsleyM.GoldsteinInheritance of varicocelesUrology6520051186118910.1016/j.urology.2004.12.057
- E.V.DahlJ.F.HerrickA vascular mechanism for maintaining testicular temperature by counter-current exchangeSurg Gynecol Obstet1081959697705
- A.W.ZorgniottiJ.MacleodStudies in temperature, human semen quality, and varicoceleFertil Steril241973854863
- M.GoldsteinJ.F.EidElevation of intratesticular and scrotal skin surface temperature in men with varicoceleJ Urol142198974374510.1016/S0022-5347(17)38874-2
- S.B.LimaM.A.CenedezeR.P.BertollaP.A.FilhoS.OehningerA.P.CedenhoExpression of the HSPA2 gene in ejaculated spermatozoa from adolescents with and without varicoceleFertil Steril8620061659166310.1016/j.fertnstert.2006.05.030
- H.HosseinifarM.SabbaghianD.NasrabadiT.ModarresiA.V.DizajH.Gourabiet alStudy of the effect of varicocelectomy on sperm proteins expression in patients with varicocele and poor sperm quality by using two-dimensional gel electrophoresisJ Assist Reprod Genet31201472572910.1007/s10815-014-0209-0
- F.KilinçF.KayaselcukC.AygunS.GuvelT.EgilmezH.OzkardesExperimental varicocele induces hypoxia inducible factor-1α, vascular endothelial growth factor expression and angiogenesis in the rat testisJ Urol17220041188119110.1097/01.ju.0000135455.97627.15
- J.D.LeeS.Y.JengT.H.LeeIncreased expression of hypoxia-inducible factor-1α in the internal spermatic vein of patients with varicoceleJ Urol17520061045104810.1016/S0022-5347(05)00417-9
- B.N.HendinP.N.KolettisR.K.SharmaA.J.ThomasA.AgarwalVaricocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacityJ Urol161199918311834
- A.AgarwalS.PrabakaranS.S.AllamaneniRelationship between oxidative stress, varicocele and infertility: a meta-analysisReprod Biomed Online12200663063310.1016/S1472-6483(10)61190-X
- W.KrzyściakM.KózkaGeneration of reactive oxygen species by a sufficient, insufficient and varicose vein wallActa Biochim Pol5820118994
- B.AltunolukE.EfeE.B.KurutasA.B.GulF.AtalayM.ErenElevation of both reactive oxygen species and antioxidant enzymes in vein tissue of infertile men with varicoceleUrol Int88201210210610.1159/000332156
- S.S.AllamaneniC.K.NaughtonR.K.SharmaA.J.ThomasJrA.AgarwalIncreased seminal reactive oxygen species levels in patients with varicoceles correlate with varicocele grade but not with testis sizeFertil Steril8220041684168610.1016/j.fertnstert.2004.04.071
- T.MostafaT.H.AnisA.El-NasharH.ImamA.OthmanVaricocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicoceleInt J Androl242001261265
- G.E.Hurtado de CatalfoA.Ranieri-CasillaF.A.MarraM.J.de AlanizC.A.MarraOxidative stress biomarkers and hormonal profile in human patients undergoing varicocelectomyInt J Androl30200751953010.1111/j.1365-2605.2007.00753.x
- I.T.KoksalY.IshakM.UstaA.DanismanE.GuntekinI.C.Bassorgunet alVaricocele-induced testicular dysfunction may be associated with disruption of blood-testis barrierArch Androl532007434810.1080/01485010600822606
- F.ComhaireA.VermeulenVaricocele sterility: cortisol and catecholaminesFertil Steril2519748895
- S.ÇayanE.AkbayM.BozluE.DorukA.YildizD.Acaret alDiagnosis of pediatric varicoceles by physical examination and ultrasonography and measurement of the testicular volumeUrol Int69200229329610.1159/000066125
- R.K.ChiouJ.C.AndersonR.K.WobigD.E.RosinskyA.MatamorosW.S.Chenet alColor Doppler ultrasound criteria to diagnose varicoceles: correlation of a new scoring system with physical examinationUrology50199795395610.1016/S0090-4295(97)00452-4
- L.DubinR.D.AmelarVaricocele size and results of varicocelectomy in selected subfertile men with varicoceleFertil Steril21197060660910.1016/S0015-0282(16)37684-1
- L.M.SarteschiR.PaoliM.BianchiniG.M.FabrisLo studio del varicocele con eco-color-DopplerG Ital Ultrasonologia419934349
- American Urological Association. Report on varicocele and infertility. An AUA Best Practice Policy and ASRM Practice Committee report, 2001. Available at: http://www.auanet.org/guidelines/archived-documents. Accessed November 2017.
- J.S.LavenL.C.HaansW.P.MaliE.R.te VeldeC.J.WensingJ.M.EimersEffects of varicocele treatment in adolescents: a randomized studyFertil Steril581992756762
- G.E.LemackR.G.UzzoP.N.SchlegelM.GoldsteinMicrosurgical repair of the adolescent varicoceleJ Urol1601998179181
- G.BogaertC.OryeG.De WinPubertal screening and treatment for varicocele do not improve chance of paternity as adultJ Urol18920132298230410.1016/j.juro.2012.12.030
- S.ÇayanS.ŞahinE.AkbayPaternity rates and time to conception in adolescents with varicocele undergoing microsurgical varicocele repair vs observation only: a single institution experience with 408 patientsJ Urol198201719520110.1016/j.juro.2017.01.066
- R.BarwellOne hundred cases of varicocele treated by the subcutaneous wire loopLancet1251885978
- A.PalomoRadical cure of varicocele by a new technique: preliminary reportJ Urol611949604607
- O.IvanissevichLeft varicocele due to reflux; experience with 4,470 operative cases in forty-two yearsJ Int Coll Surg341960742755
- P.GonteroG.PrettiF.FontanaA.ZitellaG.MarchioroB.FreaInguinal versus subinguinal varicocele vein ligation using magnifying loupe under local anesthesia: which technique is preferable in clinical practice?Urology6620051075107910.1016/j.urology.2005.05.009
- E.Sanchez de BadajozF.Diaz-RamirezC.Vara-ThorbeckEndoscopic varicocelectomyJ Endourol41990371374
- J.L.MarmarT.J.DeBenedictisD.PraissThe management of varicoceles by microdissection of the spermatic cord at the external inguinal ringFertil Steril431985583588
- M.GoldsteinB.R.GilbertA.P.DickerJ.DwoshC.GneccoMicrosurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing techniqueJ Urol148199218081811
- R.TauberN.JohnsenAntegrade scrotal sclerotherapy for the treatment of varicocele: technique and late resultsJ Urol1511994386390
- D.J.LordP.E.BurrowsPediatric varicocele embolizationTech Vasc Interv Radiol62003169175
- E.NieschlagL.HertleA.FischedickK.AbshagenH.M.BehreUpdate on treatment of varicocele: counselling as effective as occlusion of the vena spermaticaHum Reprod13199821472150
- J.L.EversJ.A.CollinsP.VandekerckhoveSurgery or embolisation for varicocele in subfertile menCochrane Database Syst Rev1200110.1002/14651858.CD000479 CD000479
- J.L.EversJ.A.CollinsSurgery or embolisation for varicocele in subfertile menCochrane Database Syst Rev3200410.1002/14651858.CD000479.pub2 CD000479
- A.AgarwalF.DeepinderM.CocuzzaR.AgarwalR.A.ShortE.Sabaneghet alEfficacy of varicocelectomy in improving semen parameters: new meta-analytical approachUrology70200753253810.1016/j.urology.2007.04.011
- I.SchauerS.MadersbacherR.JostW.A.HübnerM.ImhofThe impact of varicocelectomy on sperm parameters: a meta-analysisJ Urol18720121540154710.1016/j.juro.2011.12.084
- A.BaazeemE.BelzileA.CiampiG.DohleK.JarviA.Saloniaet alVaricocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repairEur Urol60201179680810.1016/j.eururo.2011.06.018
- T.A.Abdel-MeguidA.Al-SayyadA.TayibH.M.FarsiDoes varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trialEur Urol59201145546110.1016/j.eururo.2010.12.008
- A.C.KroeseN.M.de LangeJ.CollinsJ.L.EversSurgery or embolization for varicoceles in subfertile menCochrane Database Syst Rev10201210.1002/14651858.CD000479.pub5 CD000479
- O.YamanE.OzdilerK.AnafartaO.GöğüşEffect of microsurgical subinguinal varicocele ligation to treat painUrology552000107108
- A.ArmağanO.ErgünE.BaşT.OksayA.KoşarLong-term effects of microsurgical varicocelectomy on pain and sperm parameters in clinical varicocele patients with scrotal pain complaintsAndrologia44201161161410.1111/j.1439-0272.2011.01238.x
- S.ElzanatyC.E.JohansenEffect of microsurgical subinguinal varicocele repair on chronic dull scrotal pain in men with Grade II-III lesionsCurr Urol9201718819110.1159/000447139
- B.AltunolukH.SoylemezE.EfeO.MalkocDuration of preoperative scrotal pain may predict the success of microsurgical varicocelectomyInt Braz J Urol3620105559
- L.M.SuM.GoldsteinP.N.SchlegelThe effect of varicocelectomy on serum testosterone levels in infertile men with varicocelesJ Urol154199517521755
- C.TanrikutM.GoldsteinJ.S.RosoffR.K.LeeC.J.NelsonJ.P.MulhallVaricocele as a risk factor for androgen deficiency and effect of repairBJU Int10820111480148410.1111/j.1464-410X.2010.10030.x
- W.ZohdyS.GhaziM.ArafaImpact of varicocelectomy on gonadal and erectile functions in men with hypogonadism and infertilityJ Sex Med8201188589310.1111/j.1743-6109.2010.01974.x
- T.A.Abdel-MeguidH.M.FarsiA.Al-SayyadA.TayibH.A.MosliA.H.HalawaniEffects of varicocele on serum testosterone and changes of testosterone after varicocelectomy: a prospective controlled studyUrology8420141081108710.1016/j.urology.2014.05.029
- W.HsiaoJ.S.RosoffJ.R.PaleJ.L.PowellM.GoldsteinVaricocelectomy is associated with increases in serum testosterone independent of clinical gradeUrology8120131213121710.1016/j.urology.2013.01.060
- I.MadgarR.WeissenbergB.LunenfeldA.KarasikB.GoldwasserControlled trial of high spermatic vein ligation for varicocele in infertile menFertil Steril63199512012410.1016/S0015-0282(16)57306-3
- R.D.Shlansky-GoldbergK.N.VanArsdalenC.M.RutterM.C.SoulenZ.J.HaskalR.A.Baumet alPercutaneous varicocele embolization versus surgical ligation for the treatment of infertility: changes in seminal parameters and pregnancy outcomesJ Vasc Interv Radiol8199775976710.1016/S1051-0443(97)70657-2
- S.CayanT.C.KadiogluA.TefekliA.KadiogluS.TellalogluComparison of results and complications of high ligation surgery and microsurgical high inguinal varicocelectomy in the treatment of varicoceleUrology552000750754
- G.A.BebarsA.ZakiA.R.DawoodM.A.El-GoharyLaparoscopic versus open high ligation of the testicular veins for the treatment of varicoceleJSLS42000209213
- H.GhanemT.AnisA.El-NasharR.ShamloulSubinguinal microvaricocelectomy versus retroperitoneal varicocelectomy: comparative study of complications and surgical outcomeUrology6420041005100910.1016/j.urology.2004.06.060
- M.WatanabeA.NagaiN.KusumiH.TsuboiY.NasuH.KumonMinimal invasiveness and effectivity of subinguinal microscopic varicocelectomy: a comparative study with retroperitoneal high and laparoscopic approachesInt J Urol12200589289810.1111/j.1442-2042.2005.01142.x
- A.ZucchiL.MeariniE.MeariniE.CostantiniV.BiniM.PorenaTreatment of varicocele: randomized prospective study on open surgery versus Tauber antegrade sclerotherapyJ Androl26200532833210.2164/jandrol.04143
- A.ZucchiL.MeariniE.MeariniF.FiorettiV.BiniM.PorenaVaricocele and fertility: relationship between testicular volume and seminal parameters before and after treatmentJ Androl27200654855110.2164/jandrol.05200
- A.M.Al-KandariH.ShabaanH.M.IbrahimY.H.ElshebinyA.A.ShokeirComparison of outcomes of different varicocelectomy techniques: open inguinal, laparoscopic, and subinguinal microscopic varicocelectomy: a randomized clinical trialUrology69200741742010.1016/j.urology.2007.01.057
- S.Al-SaidA.Al-NaimiA.Al-AnsariN.YounisA.ShamsodiniK.A-sadiqet alVaricocelectomy for male infertility: a comparative study of open, laparoscopic and microsurgical approachesJ Urol180200826627010.1016/j.juro.2008.03.050
- A.FayezK.M.El ShantalyM.AbbasS.HauserS.C.MüllerA.FathyComparison of inguinal approach, scrotal sclerotherapy and subinguinal antegrade sclerotherapy in varicocele treatment: a randomized prospective studyUrol. Int85201020020310.1159/000316338
- S.CayanA.KadiogluI.OrhanE.KandiraliA.TefekliS.TellalogluThe effect of microsurgical varicocelectomy on serum follicle stimulating hormone, testosterone and free testosterone levels in infertile men with varicoceleBJU Int84199910461049
- A.JungwirthC.GögüsG.HauserA.GomahrN.SchmellerW.Aulitzkyet alClinical outcome of microsurgical subinguinal varicocelectomy in infertile menAndrologia3320017174
- K.M.KamalK.JarviA.ZiniMicrosurgical varicocelectomy in the era of assisted reproductive technology: influence of initial semen quality on pregnancy ratesFertil Steril75200110131016
- M.TestiniS.MinielloG.PiccinniB.Di VenereG.LissidiniE.EspositoMicrosurgical treatment of varicocele in outpatients using the subinguinal approachMinerva Chir562001655659
- P.PerimenisS.MarkouK.GyftopoulosA.AthanasopoulosG.BarbaliasEffect of subinguinal varicocelectomy on sperm parameters and pregnancy rate: a two-group studyEur Urol392001322325
- S.ÇayanF.ErdemirI.OzbeyP.J.TurekA.KadioğluS.TellaloğluCan varicocelectomy significantly change the way couples use assisted reproductive technologies?J Urol167200217491752
- R.KumarN.P.GuptaSubinguinal microsurgical varicocelectomy: evaluation of the resultsUrol Int71200336837210.1159/000074087
- I.OrhanR.OnurA.SemerciözF.FirdolasA.ArdicogluI.T.KoksalComparison of two different microsurgical methods in the treatment of varicoceleArch Androl512005213220
- S.ÇayanD.AcarS.ÜlgerE.AkbayAdolescent varicocele repair: long-term results and comparison of surgical techniques according to optical magnification use in 100 cases at a single university hospitalJ Urol17420052003200710.1097/01.ju.0000176488.44895.7b
- J.LibmanK.JarviK.LoA.ZiniBeneficial effect of microsurgical varicocelectomy is superior for men with bilateral versus unilateral repairJ Urol17620062602260510.1016/j.juro.2006.07.161
- J.M.BomanJ.LibmanA.ZiniMicrosurgical varicocelectomy for isolated asthenospermiaJ Urol18020082129213210.1016/j.juro.2008.07.046
- A.F.Abdel-MaguidI.OthmanMicrosurgical and nonmagnified subinguinal varicocelectomy for infertile men: a comparative studyFertil Steril9420102600260310.1016/j.fertnstert.2010.03.063
- K.BakerJ.McGillR.SharmaA.AgarwalE.SabaneghJr.Infertility pregnancy after varicocelectomy: impact of postoperative motility and DFIUrology81201376076610.1016/j.urology.2012.12.005
- E.B.NasrM.BinhazzaaT.AlmontP.RischmannM.SoulieE.HuygheSubinguinal microsurgical varicocelectomy vs. percutaneous embolization in infertile men: Prospective comparison of reproductive and functional outcomes2720171110.1186/s12610-017-0055-x
- S.M.TanF.C.NgT.RavintharanP.H.LimH.C.ChngLaparoscopic varicocelectomy: technique and resultsBr J Urol751995523528
- M.F.MiladT.A.ZeinE.A.HusseinF.M.AyyatM.P.SchneiderG.R.SantLaparoscopic varicocelectomy for infertility. An initial report from Saudi ArabiaEur Urol291996462465
- N.ZampieriV.ZuinM.CorroppoloC.ChironiR.M.CervellioneF.S.CamoglioVaricocele and adolescents: semen quality after 2 different laparoscopic proceduresJ Androl28200772773310.2164/jandrol.107.002600
- N.ZampieriA.MantovaniA.OttolenghiF.S.CamoglioTesticular catch-up growth after varicocelectomy: does surgical technique make a difference?Urology73200928929210.1016/j.urology.2008.07.039
- J.M.FergusonI.N.GillespieN.ChalmersR.A.EltonT.B.HargreavePercutaneous varicocele embolization in the treatment of infertilityBr J Radiol68199570070310.1259/0007-1285-68-811-700
- G.NabiS.AsterlingsD.R.GreeneR.L.MarshPercutaneous embolization of varicoceles: outcomes and correlation of semen improvement with pregnancyUrology63200435936310.1016/j.urology.2003.09.026
- C.BilreiroP.DonatoJ.F.CostaA.AgostinhoV.CarvalheiroF.Caseiro-AlvesVaricocele embolization with glue and coils: a single center experienceDiagn Interv Imaging98201752953410.1016/j.diii.2017.01.006
- R.D.BiggersD.W.SoderdahlThe painful varicoceleMil Med1461981440441
- C.O.YeniyolA.TunaH.YenerN.ZeyrekA.TilkiHigh ligation to treat pain in varicoceleInt Urol Nephrol3520036568
- M.E.Abd EllatifW.AskerA.AbbasA.NegmM.Al-KataryH.El-Kaffaset alVaricocelectomy to treat pain, and predictors of success: a prospective studyCurr Urol62012333610.1159/000338867
- A.C.PetersonR.S.LanceH.E.RuizOutcomes of varicocele ligation done for painJ Urol15919981565156710.1097/00005392-199805000-00043
- K.KarademirT.SenkulK.BaykalF.AteşC.IşeriD.ErdenEvaluation of the role of varicocelectomy including external spermatic vein ligation in patients with scrotal painInt J Urol12200548448810.1111/j.1442-2042.2005.01063.x
- H.J.ParkS.S.LeeN.C.ParkPredictors of pain resolution after varicocelectomy for painful varicoceleAsian J Androl13201175475810.1038/aja.2010.87
- H.T.KimP.H.SongK.H.MoonMicrosurgical ligation for painful varicocele: effectiveness and predictors of pain resolutionYonsei Med J53201214514610.3349/ymj.2012.53.1.145
- H.SoylemezN.PenbegülM.AtarY.BozkurtA.A.SancaktutarB.AltunolukComparison of laparoscopic and microscopic subinguinal varicocelectomy in terms of postoperative scrotal painJSLS16201221221710.4293/108680812X13427982376220
- Y.W.ParkJ.H.LeePreoperative predictors of varicocelectomy success in the treatment of testicular painWorld J Mens Health312013586610.5534/wjmh.2013.31.1.58
- H.ElbardisiA.AgarwalA.MajzoubS.A.SaidH.AlnawasraK.Khalafallaet alDoes the number of veins ligated during microsurgical subinguinal varicocelectomy impact improvement in pain post-surgery?Transl Androl Urol6201726427010.21037/tau.2017.03.56
- S.KachrilasE.PopovA.BourdoumisW.AkhterM.El HowairisI.Aghawayset alLaparoscopic varicocelectomy in the management of chronic scrotal painJSLS18e201420140030230410.4293/JSLS.2014.00302
- D.ScherrM.GoldsteinComparison of bilateral versus unilateral varicocelectomy in men with palpable bilateral varicocelesJ Urol1621999858810.1097/00005392-199907000-00021