Abstract
Objectives To evaluate different laboratory assessments of oxidative stress (OS) in semen and identify a cost-efficient and highly sensitive instrument capable of providing a comprehensive measure of OS in a clinical setting, as early intervention and an accurate diagnostic test are important because they help maintain a balance of free radicals and antioxidants; otherwise, excessive OS could lead to sperm damage and result in male infertility.
Materials and methods A systematic literature search was performed through a MedLine database search using the keywords ‘semen’ AND ‘oxygen reduction potential’. We also reviewed the references of retrieved articles to search for other potentially relevant research articles and additional book chapters discussing laboratory assessments for OS, ranging from 1994 to 2017. A total of 29 articles and book chapters involving OS-related laboratory assays were included. We excluded animal studies and articles written in languages other than English.
Results Direct laboratory techniques include: chemiluminescence, nitro blue tetrazolium, cytochrome C reduction test, fluorescein probe, electron spin resonance and oxidation–reduction potential (ORP). Indirect laboratory techniques include: measurement of Endtz test, lipid peroxidation, chemokines, antioxidants/micronutrients/vitamins, ascorbate, total antioxidant capacity, or DNA damage. Each of these laboratory techniques has its advantages and disadvantages.
Conclusion Traditional OS laboratory assessments have their limitations. Amongst the prevalent laboratory techniques, ORP is novel and better option as it can be easily used in a clinical setting to provide a comprehensive review of OS. However, more studies are needed to evaluate its reproducibility across various laboratory centres.
Introduction
Oxidative stress (OS) is the result of an imbalance between the formation of reactive oxygen species (ROS) and the inability of the available antioxidants to neutralise the excessive production of ROS. OS results in lipid peroxidation, protein changes and DNA damage and sperm death [Citation1]. OS is one of the most common aetiologies affecting 30–80% of men with infertility [Citation2,Citation3]. Studies have shown that OS is one of the major causes of male infertility, especially for those who have unexplained and idiopathic male infertility [Citation1]. OS can not only affect spermatozoa but also can potentially have consequences on a systematic level such as decreasing the amount of testosterone or LH [Citation4]. Increased OS also directly damages sperm DNA, thus compromising the paternal genomic contribution to the embryo [Citation5]. Due to these characteristics, OS in male infertility can lead to serious health risks if not carefully controlled over time. There are several laboratory tests available to measure OS; however, many of these are either expensive or cumbersome to carry out as routine diagnostic tests. Based on the current literature, there is a greater need to review available tests and identify the need for the development of a novel approach to reliably measure OS.
The present paper will aim to: (i) review traditional laboratory assessments including direct and indirect techniques relevant to OS in male infertility, along with advantages and disadvantages of each technique, (ii) provide detailed description on the most prevalent laboratory techniques currently used in the laboratories, and (iii) provide insight for future directions to see how laboratory assessments for measuring OS could be more effective in the clinical setting.
Relationship between OS and principles of laboratory techniques
The rationale for various laboratory techniques used to measure OS are a result of direct measurement of ROS or indirect measurements through oxidised products of ROS production. Direct laboratory techniques measure OS or free radicals such as ROS and reactive nitrogen species, whereas indirect laboratory techniques measure lipid peroxidation, antioxidants, cofactors, or other end products secondary to ROS production. More specifically, indirect measurements could be an accumulative result of oxidised products resulting from sources of ROS such as the oxidised form of nicotinamide adenine dinucleotide (NADPH)-oxidase in the sperm, the reduced form of NAD (NADH)-dependent oxidoreductase in mitochondria, or leucocytospermia [Citation6].
Available laboratory techniques
There are a number of laboratory techniques for measuring OS. Traditional OS laboratory techniques include direct and indirect assessment of OS. Direct laboratory techniques include chemiluminescence, nitro blue tetrazolium (NBT), cytochrome C reduction test, fluorescein probe, electron spin resonance and oxidation reduction potential (ORP). Indirect laboratory techniques include measurement of Endtz test, lipid peroxidation, chemokines, antioxidants/ micronutrients/vitamins, ascorbate, total antioxidant capacity (TAC), or DNA damage. Each of these laboratory techniques has its advantages and disadvantages, as shown in and .
Table 1 Advantages and disadvantages of direct measurement of ROS in semen.
Table 2 Advantages and disadvantages of indirect measurement of ROS in semen.
Limitations of traditional laboratory measurement of OS
More than one study has indicated that there has been misclassification of fertility due to lower reference values set by the WHO fifth edition 2010 guidelines [Citation20]. Other contributing factors could result from limitations of routine semen analysis [Citation8]. Thus, an accurate, cost-efficient, highly sensitive, and comprehensive OS laboratory assessment is crucial for a male infertility routine clinical laboratory test.
Common laboratory techniques available for OS measurement
Although there is no general cure for OS-induced male infertility, preventative actions such as having a cost-efficient and comprehensive laboratory test can be carried out for early intervention. These tests can potentially lower OS related symptoms such as increased lipid peroxidation, DNA fragmentation, and poor antioxidant reserves as observed in varicocele, idiopathic/unexplained infertility and asthenozoospermia. Amongst those laboratory assessments discussed above, some of the prevalent ones include the chemiluminescence assay, TAC assay, and ORP test.
Measurement of ROS by chemiluminescence
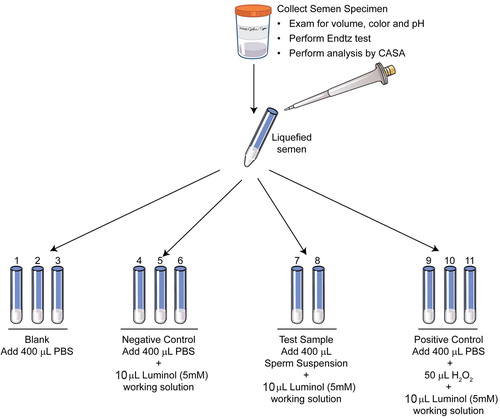
Chemiluminescence is extremely sensitive and reacts with a variety of ROS at neutral pH. In the presence of a suitable catalyst, this test utilises the emission of light to measure extracellular and intracellular ROS using luminol or lucigenin as the probe [Citation7]. Measurement of ROS by chemiluminescence assay using luminol is a more common method to measure global ROS. A 5 mM working solution of luminol is used. The test includes blank (PBS only), negative (PBS + luminol), positive (H2O2 + luminol) and a test sample (liquefied semen sample). ROS is measured for 15 min and the results are expressed as relative light units/s/106 sperm (–) [Citation7]. The reference value for normal ROS obtained by this method is <102 relative light units/s/106 sperm/mL. Some of the limitations of this assay are: it cannot be performed in patient samples with low volume because a 800 µL sample is required to run the sample in duplicate [Citation7] and it cannot be measured in frozen samples. Whilst this test has been sensitive, confounding variables such as the presence of leucocytes could interfere with laboratory result [Citation2]. In such cases, the use of magnetic beads coated with a monoclonal antibody or the use of fluorescent probes have been suggested [Citation2].
Fig. 1 External view of the luminometer connected to the monitor for real-time display of ROS production on the monitor screen.

Measurement of total antioxidants in seminal plasma
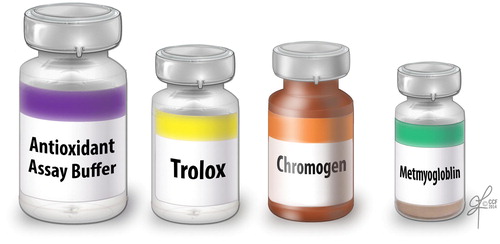
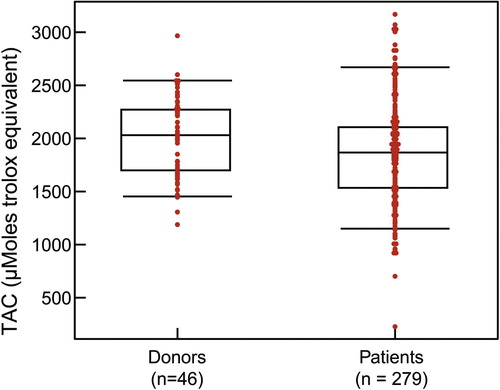
The TAC assay measures the total antioxidant available in the sample – both enzymatic and non-enzymatic, and presence of macromolecules. The TAC is measured in the clear seminal plasma after removing all the cellular components from the seminal ejaculate. TAC can be measured using a TAC assay kit. The components consist of assay buffer, standard Trolox, chromogen and metmyoglobin (). Hydrogen peroxide is used to initiate the reaction. TAC inhibits ABTS (2,2′-azino-di-[3-ethylbenzthiazolinesulfonate]) to ABTS+ by metmyoglobin [Citation16]. Under the induced reaction conditions, the antioxidants in the seminal plasma suppress the absorbance at 750 nm to a degree that is proportional to the concentration of antioxidant. By comparing to standard Trolox, a water-soluble tocopherol analogue, the amount of antioxidant is measured in the sample by a colorimeter [Citation16]. The unit of measurement of TAC is micromoles Trolox equivalent. The reference value for normal antioxidant value in a sample is >1950 micromoles of Trolox equivalent. Lower TAC values are indicative of increased OS or reduced ability of antioxidants to scavenge the formation of ROS in semen samples from infertile men (). Whilst this test is a rapid colorimetric method and able to measure total amount of antioxidants available in seminal plasma sample, it does not measure any enzymatic antioxidants [Citation3,Citation17]. Similar to other traditional diagnostic tests, this test is expensive to be incorporated in a clinical setting as a routine diagnostic laboratory test for male infertility.
Measurement of OS by ORP
ORP is a novel direct measurement of OS or redox imbalance in biological samples [Citation21]. It is correlated with illness, injury, and severity in trauma patients [Citation22]. It involves the measurement of the transfer of electrons from a reductant (or antioxidant) to an oxidant, which measures the existing balance between total oxidants and reductants (redox potential) in a biological system [Citation21]. It is a galvanostat-based technique that measures redox potential using the Male Infertility Oxidative System (MiOXSYS®; Aytu BioScience Inc., Englewood, CO, USA). It consists of a MiOXSYS analyser and the sensor strip. In all, 30 µL of completely liquefied semen sample is loaded on the sample port and measured for ∼4 min (–). It is measured in millivolts (mV). The ORP values are normalised by the sperm concentration and expressed as mV/106 sperm/mL [Citation21]. ORP values >1.37 mV/106 sperm/mL are indicative of OS. Unlike assessment of a single marker, ORP can function as an advanced and independent test for semen quality in male infertility [Citation23].
Fig. 6 Measurement of ORP by the MiOXSYS system. (A) MiOXSYS analyser showing the sensor socket and the sensor module and (B) sensor showing the reference cell and the sample port where the sample is loaded.
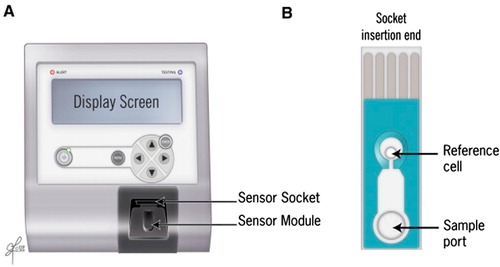
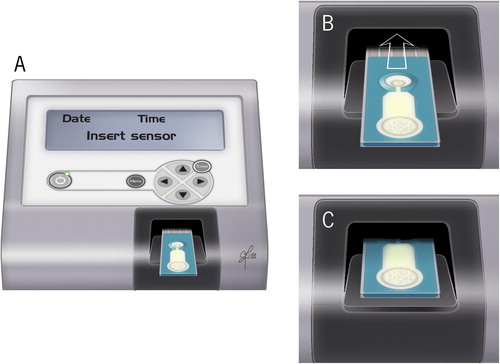
Fig. 7 MiOXSYS system (A) placing of the sensor strip on the sensor socket (B-C) inserting the sensor in the sensor port.
Current evidence suggests that measurement of ORP can serve as a standalone test or a combination of laboratory measures of semen parameters such as concentration, motility, total sperm concentration can further improve the specificity and positive predictive value of this test [Citation21,Citation24Citation[25]Citation[26]–Citation27]. ORP’s advantages and disadvantages are shown in
Table 3 Advantages and disadvantages of ORP in semen.
In a small clinical setting, semen analysis is not cost effective and traditional laboratory assessments have their own limitations, especially at the WHO threshold values [Citation24,Citation27]. In the case of semen analysis, sperm number and motility carry the most weight to assess male fertility [Citation25]. Different probes detect free radicals or oxidised products at different endpoints and locations of the spermatozoa. Regardless of the specific technique adopted by various laboratories or clinicians, probe selections also play a role in terms of the specific free radicals that could be detected [Citation6,Citation28]. For example, chemiluminescence can be detected both by luminol and lucigenin; luminol can detect both extracellular and intracellular free radicals, whilst lucigenin can only detect extracellular free radicals, specifically superoxide anion [Citation6]. Luminol seems to be a better option, as it can measure both extracellular and intracellular free radicals or global measure of ROS, but lucigenin probe has better sensitivity specifically for O2 − production [Citation6].
Similar to chemiluminescence, most of the available tests also have one or more probes in terms of specificity and sensitivity. Differences in the selection of these probes also tend to have different sensitivity and specificity. Early detection of OS could provide a valuable therapeutic option in the management and treatment of male infertility, an accurate, user-friendly, and cost-efficient test would be much appreciated in a clinical setting. Through comparing and contrasting between chemiluminescence and ORP amongst the latest laboratory techniques, ORP is a better option as it provides a direct measurement of OS and can identify both normal and abnormal semen parameters and differentiate fertile men from infertile patients [Citation23,Citation25].
Some of the important characteristics when evaluating laboratory tests include the types of samples each test can measure, time to complete the test, sample volume, different confounding factors that could interfere with the test assay, and cost to conduct the test. An example of such comparison between the chemiluminescence and ORP test is shown in . Furthermore, ORP is a cost-effective alternate to other tests such as the computer-assisted semen analyser that requires expensive equipment and expertise or measuring ROS by chemiluminescence assay or DNA fragmentation by flow cytometry. In ORP the cost is mainly associated with the disposable sensor strips.
Table 4 Comparisons between chemiluminescence and ORP.
In conclusion, current methods of assessing OS have limitations such as being laborious, time consuming, expensive, and measuring only a single marker of OS at a given time. In comparison ORP can be used in a small clinical setting as a standalone test or in conjunction with semen parameters, it is quick and user friendly and provides a comprehensive review of OS. However, additional studies are necessary to evaluate its reproducibility across various laboratory centres.
Future research
Looking at the advantages, disadvantages, limitations, along with parameters used to evaluate each laboratory technique, the ORP test is a promising new andrology laboratory test. ORP is able to distinguish not only abnormal and normal semen quality, but also discriminate between semen from fertile and infertile patients with specific thresholds [Citation24,Citation25,Citation27]. However, there is a strong need for the test to be validated in different clinical settings across the globe to establish the reference values and hence a stronger evidence for its clinical efficacy [Citation27]. Furthermore, there is also a need to look for more laboratory tests measuring OS of sperm functions such as sperm chromatin structure assay (SCSA), terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL), comet, sperm chromatin dispersion assay. The threshold value to differentiate infertile men with DNA damage varies amongst different Andrology laboratory settings because of differences in study populations, sperm preparation methods, and different assisted reproductive techniques (ART) used [Citation18,Citation29]. Future research could look into strategies to establish a more rigorous threshold for DNA fragmentation that tailors towards the specific laboratory setting.
Conflict of interest
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- A.AgarwalD.DurairajanayagamJ.HalabiJ.PengM.Vazquez-LevinProteomics, oxidative stress and male infertilityReprod Biomed Online2920143258
- J.AitkenH.FisherReactive oxygen species generation and human spermatozoa: the balance of benefit and riskBioEssays161994259267
- A.AgarwalS.GuptaS.SikkaThe role of free radicals and antioxidants in reproductionCurr Opin Obstet Gynecol182006325332
- R.HenkelInfection in InfertilityS.J.ParekattilA.AgarwalMale infertility contemporary clinical approaches, andrology, ART & antioxidants2012SpringerNew York261272
- K.TremellenOxidative stress and male infertility–a clinical perspectiveHum Reprod Update142008243258
- D.BenjaminR.K.SharmaA.MoazzamA.AgarwalMethods for the detection of ROS in human sperm samplesA.AgarwalR.J.AitkenJ.G.AlvarezStudies on men’s health and fertility2012Humana PressNew York257273
- A.AgarwalS.GuptaR.SharmaReactive oxygen species (ROS) measurementA.AgarwalS.GuptaR.SharmaAndrological: a laboratory guide2016Springer International PublishingNew York155163
- A.AgarwalA.MajzoubLaboratory tests for oxidative stressIndian J Urol332017199206
- N.EsfandiariR.K.SharmaR.A.SalehA.J.ThomasJrA.AgarwalUtility of the nitroblue tetrazolium reduction test for assessment of reactive oxygen species production by seminal leukocytes and spermatozoaJ Androl242003862870
- S.I.DikalovD.G.HarrisonMethods for detection of mitochondrial and cellular reactive oxygen speciesAntioxid Redox Signal202014372382
- O.I.FarahL.CuilingW.JiaojiaoZ.HuipingUse of fluorescent dyes for readily recognizing sperm damageJ Reprod Infertil142013120125
- R.J.AitkenJ.P.BrindleAnalysis of the ability of three probes targeting the outer acrosomal membrane or acrosomal contents to detect the acrosome reaction in human spermatozoaHum Reprod8199316631669
- M.KohnoApplications of electron spin resonance spectrometry for reactive oxygen species and reactive nitrogen species researchJ Clin Biochem Nutr472010111
- S.ReuterS.C.GuptaM.M.ChaturvediB.B.AggarwalOxidative stress, inflammation, and cancer: how are they linked?Free Radic Biol Med49201016031616
- Schröder, JM. Purification of chemokines from natural sources. In: Proudfoot AE, Wells TNC, Power CA (eds.), Methods in molecular biology, vol. 138: Chemokine Protocols. Totowa, NJ: Humana Press Inc.; 2000: pp. 1–10.
- A.AgarwalS.GuptaR.SharmaAntioxidant measurement in seminal plasma by TAC assayA.AgarwalS.GuptaR.SharmaAndrological evaluation of male infertility: a laboratory guide2016Springer International PublishingNew York171179
- S.RoychoudhuryR.SharmaS.SikkaA.AgarwalDiagnostic application of total antioxidant capacity in seminal plasma to assess oxidative stress in male factor infertilityJ Assist Reprod Genet332016627635
- R.K.SharmaE.SabaneghR.MahfouzS.GuptaA.ThiyagarajanA.AgarwalTUNEL as a test for sperm DNA damage in the evaluation of male infertilityUrology76201013801386
- S.BishtM.FaiqM.TolahunaseR.DadaOxidative stress and male infertilityNat Rev Urol142017470485
- K.S.MurrayA.JamesJ.B.McGeadyM.L.ReedW.W.KuangA.K.NangiaThe effect of the new 2010 World Health Organization criteria for semen analyses on male infertilityFertil Steril98201214281431
- A.AgarwalS.GuptaR.SharmaOxidation-reduction potential measurement in ejaculated semen samplesA.AgarwalS.GuptaR.SharmaAndrological evaluation of male infertility: a laboratory guide2016Springer International PublishingNew York165170
- L.T.RaelR.Bar-OrR.M.AumannD.S.SloneC.W.MainsD.Bar-OrOxidation-reduction potential and paraoxonase-arylesterase activity in trauma patientsBiochem Biophys Res Commun3612007561565
- A.AgarwalS.RoychoudhuryK.B.BjugstadC.L.ChoOxidation-reduction potential of semen: what is its role in the treatment of male infertility?Ther Adv Urol82016302318
- A.AgarwalS.M.WangClinical relevance of oxidation-reduction potential in the evaluation of male infertilityUrology10420178489
- M.ArafaA.AgarwalS.Al SaidA.MajzoubR.SharmaK.B.Bjugstadet alSemen quality and infertility status can be identified through measures of oxidation-reduction potentialAndrologia201710.1111/and.12881 [Epub ahead of print]
- A.AgarwalR.SharmaS.RoychoudhuryS.Du PlessisE.SabaneghMiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasmaFertil Steril1062016566573
- A.AgarwalM.ArafaR.ChandrakumarA.MajzoubS.AlSaidH.ElbardisiA multicenter study to evaluate oxidative stress by oxidation-reduction potential, a reliable and reproducible methodAndrology52017939945
- A.AgarwalS.S.AllamaneniT.M.SaidChemiluminescence technique for measuring reactive oxygen speciesReprod Biomed Online92004466468
- Z.ZhangL.ZhuH.JiangH.ChenY.ChenY.DaiSperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: a meta-analysisJ Assist Reprod Genet3220151726