Abstract
Objective: To evaluate various methods of operative sperm retrieval in men with non-obstructive azoospermia (NOA) and to determine the optimal surgical approach in terms of effectiveness, morbidity, and complications.
Materials and methods: PubMed and Cochrane databases were searched to identify five recent reviews and meta-analyses evaluating outcomes for sperm retrieval in men with NOA.
Results and Conclusion: Micro-TESE is the most efficient method for retrieving sperm but requires special expertise and can be traumatic for the testes. Conventional biopsies are twice more likely to retrieve sperm than fine-needle aspiration. Testicular aspiration performed by multiple passes into the testis is traumatic and is not efficient for sperm retrieval. Needle-aspiration biopsy and open real-time testicular mapping by the single seminiferous tubule technique can offer less traumatic methods for sperm retrieval, which can be tried before proceeding to micro-TESE. The first attempt at sperm retrieval is the best chance the patient has and should combine various techniques sequentially to give the highest chance of success with the least morbidity.
Abbreviations:
- AZF(a)(b)(c)
- azoospermia factor (a) (b) (c)
- (O)FNA
- (open) fine-needle aspiration
- ICSI
- intracytoplasmic sperm injection
- IVF
- in vitro fertilisation
- MeSH
- medical subject heading
- NAB
- needle aspiration biopsy;
- (N)OA
- (non-)obstructive azoospermia
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- SRR
- sperm retrieval rate
- SSSSR
- single-session staged sperm retrieval
- SST
- single seminiferous tubule
- TESA
- testicular sperm aspiration
- (c) (micro-) (n) TESE
- (conventional) (microdissection) (needle) testicular sperm extraction
Introduction
The advent of intracytoplasmic sperm injection (ICSI) has revolutionised the management of male infertility and made it possible for even an azoospermic man to father a child using sperm retrieved from his epididymis or testis. Operative sperm retrieval has become a routine procedure in all in vitro fertilisation (IVF) clinics. However, even though a variety of techniques have been described, there is confusion about which method of retrieval is best suited for a given patient, and most clinics tend to apply the same technique to all patients, which may not be in the best interest of a specific patient.
Operative sperm retrieval is indicated in men with obstructive azoospermia (OA) if reconstruction is not possible, or has failed. In men with OA, sperm may be retrieved percutaneously by percutaneous epididymal aspiration (PESA) [Citation1] or by open surgical procedures, e.g. microsurgical epididymal sperm aspiration (MESA) [Citation2], or the simpler procedure of open fine-needle aspiration (OFNA) that does not need an operating microscope [Citation3], or by a single open testicular biopsy. In any case, sperm retrieval is easy and assured.
The greater challenge is sperm retrieval in men with non-OA (NOA): only some of these men will have a few sperm in the testes, and the distribution of these scanty sperm may be multi-focal or very localised, necessitating different sperm-retrieval techniques.
The purpose of the present review is to critically evaluate the findings and conclusions of recent reviews and meta-analyses on operative sperm retrieval in NOA, so as to develop a customised approach that would enable the clinician to choose from the range of techniques described and offer his patient the best chance of sperm retrieval with the least morbidity and complications.
Materials and methods
A comprehensive search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines using the PubMed and Cochrane databases from 2011 to 2017. The following keywords were used: ‘testicular sperm extraction’, ‘azoospermia’, ‘sperm retrieval’, and the medical subject heading (MeSH) phrases ‘azoospermia[MeSH]’ AND ‘sperm retrieval[MeSH]’ were included.
Papers were excluded if they did not deal with sperm retrieval in NOA, or lacked detailed description of the techniques or comparative outcomes.
Outcomes of interest were: sperm retrieval techniques, sperm retrieval rates (SRRs), prognostic factors, and complications. Inclusion criteria were meta-analyses comparing different techniques or review articles providing detailed comparisons or descriptions of various sperm retrieval procedures.
Results
Literature search
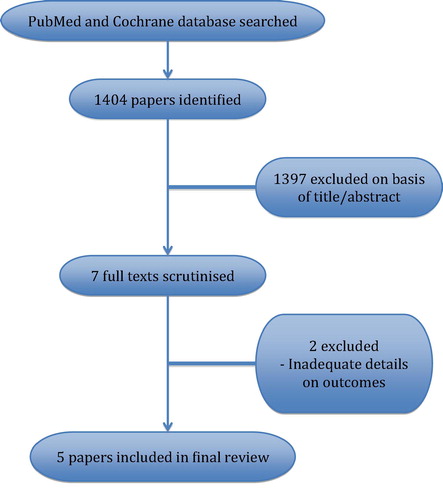
The search strategy identified 1404 papers, of which 1397 were excluded on the basis of title or abstract. Seven full texts were scrutinised [Citation4Citation[5]Citation[6]Citation[7]Citation[8]Citation[9]–Citation10] and two reviews [Citation4,Citation6] were excluded because of incomplete data on comparative sperm retrieval outcomes (). The characteristics of the five papers included in this review are summarised in .
Table 1 Characteristics of the studies included in the review.
Techniques of sperm retrieval
Various percutaneous and open procedures have been described, some requiring the use of an operating microscope. These are summarised in .
Table 2 Techniques for operative sperm retrieval.
Efficacy of sperm retrieval
All reviews found microdissection testicular sperm extraction (micro-TESE) to have the highest SRRs. The systemic review by Deruyver et al. [Citation7] reported SRRs of 16.7–45% by conventional TESE (cTESE), vs 42.9% to 63% with micro-TESE. However, they found that micro-TESE was superior only in men with Sertoli-cell-only syndrome, and that there was no statistical difference in SRR for men with maturation arrest. The meta-analysis by Bernie et al. [Citation9] compared SRR by testicular sperm aspiration (TESA), cTESE and micro-TESE. They found that cTESE was twice more likely to find sperm than TESA, and that micro-TESE was 1.5-times more likely to find sperm as compared to cTESE.
Complications of operative sperm retrieval
Apart from the routine surgical complications of infection, bleeding and haematoma the main concern was testicular damage, as all procedures involve extensive sampling of the testis. This damage is evident both anatomically and physiologically.
Anatomical changes seen on ultrasonography include hypoechoic areas and calcification [Citation11]. Amer et al. [Citation12] reported acute and chronic changes, and temporary segmental devascularisation, after both cTESE and micro-TESE during serial ultrasonography over 6 months.
Physiological damage manifests as a fall in testosterone levels and failure to retrieve sperm in subsequent attempts [Citation13]. The fall in testosterone may take 6–18 months to recover [Citation8]. De novo hypogonadism was reported in 16% men in one series [Citation14] and some men with very small testes may remain permanently hypogonadal. Micro-TESE appears to cause less damage than multiple biopsies by cTESE [Citation15].
Predictors of successful sperm retrieval
There are conflicting reports on predictors of sperm retrieval. Some studies reported lower SRRs with high FSH [Citation16], and small testes [Citation17], whilst others found no correlation [Citation18]. One review [Citation8] reported the use of multivariate analysis to predict sperm retrieval but even then only achieved a sensitivity and specificity of 78% and 76.3%, respectively. However, none of these parameters could predict the presence or absence of sperm with 100% accuracy. It may be possible to find sperm in a very tiny testis with very high FSH and low testosterone, and not find sperm in a large testis with normal FSH!
Sperm have been found in 30–70% of men with Klinefelter syndrome [Citation19], and in men with azoospermia after orchidopexy (SRR 63.1%) or radiotherapy/chemotherapy (SRR 50%) [Citation8].
About 50–60% of men with azoospermia factor c (AZFc) microdeletions will have sperm retrievable from the testes [Citation20]; however, sperm will not be found in men with complete deletions in the AZFa or AZFb regions of the Y chromosome.
Testicular histology has some predictive value with the highest SRR in hypospermatogenesis at 73–100% [Citation10], 40% in maturation arrest, and only 20% in Sertoli-cell-only syndrome [Citation21].
Medical treatment prior to sperm retrieval
There is some evidence that men with NOA may have improved sperm retrieval after hormonal stimulation. Hormone therapy for men with NOA seems paradoxical, as most of them already have elevated gonadotrophins. But perhaps there is scope for super-stimulating the flagging testis to function a little better. Thus, Hussein et al. [Citation22] reported higher sperm retrieval after using clomiphene or human chorionic gonadotrophin to raise testosterone to the upper quartile and to elevate FSH by 1.5-times. Shiraishi et al. [Citation23] could find sperm after 6 months of high doses of gonadotrophins in 20% of men who had had a previous negative micro-TESE compared to 0% in those who had repeat micro-TESE without any hormone therapy.
The evidence that hormone therapy helps men with NOA undergoing sperm retrieval is very limited and one large retrospective study of 1054 men did not find any benefit of hormone therapy [Citation24]. However, as these are difficult cases with a guarded prognosis, clinicians do whatever they can to improve chances even a small bit. Accordingly, we consider hormone therapy in those men who have testosterone levels in the lower quartile, and whose gonadotrophins are not too elevated. If testosterone is >400 ng/dL, or if gonadotrophins are >1.5-times the upper limit, then we believe that the testes are already adequately stimulated and additional stimulation will not help.
Discussion
Method of sperm retrieval
TESA/testicular FNA [Citation25] is akin to a fine-needle cytology examination. A 20- or 22-G scalp vein needle is pushed through the testis in various directions whilst suction is applied with a 10 or 20 mL syringe. The aspirate, consisting of fluid and tiny pieces of testicular tissue is inspected for sperm. Whilst the procedure seems innocuous, it is actually very traumatic to the testis, as the multiple passages in different directions macerate the tissue and causes intra-testicular bleeding. One author reported making 50–100 passes with an 18-G needle! [Citation13]. The damage caused by this is seen clinically in the finding that in 11% of cases where sperm were present during the first TESA procedure, no sperm were found during a repeat procedure [Citation13]. A study on the rat testis showed that TESA led to high antibody titres, increased germ cell apoptosis, and extensive fibrosis of the parenchyma [Citation26]. Thus, TESA is inefficient (low SRR) and traumatic, and hence is not recommended even though it is widely used by gynaecologists.
cTESE involves multiple conventional open biopsies [Citation27]. Since a couple of careful, conventional biopsies would not cause significant damage, and can retrieve sperm in some men with NOA, it can be a step prior to proceeding to micro-TESE. However, multiple conventional biopsies can cause significant damage, and yet not be efficient in exploring the entire testis; hence cTESE is unsuitable for men with very localised spermatogenesis.
Needle aspiration biopsy (NAB)/needle TESE (nTESE) [Citation4] is a percutaneous method for obtaining a testicular biopsy. An 18-G scalp vein needle is pushed into the testis and suction is applied with a 10-mL syringe. The needle is moved back and forth in one direction only. The scalp vein tubing is then clamped near the syringe to prevent tissue from being sucked into the syringe and the needle is slowly withdrawn. When the needle exits the testis, a thick strand of testicular tissue is seen. This is grasped with a pair of microsurgical, non-serrated forceps and more tissue is pulled out from the testis. The clamp is then released and the tubing is flushed with air to deliver another piece of tissue from the needle. Thus, a large piece of testicular tissue, equal to an open biopsy, is obtained. Hence, this NAB procedure is also termed nTESE, as compared to open cTESE. Whilst this may seem the same as TESA, the NAB procedure is significantly different. The goal here is to percutaneously acquire a proper piece of testicular tissue, equal to an open biopsy, and not just an aspirate. Hence, a larger needle is used and it is passed only in one direction, as the goal is not to macerate the testis but to aspirate a core of seminiferous tubules. As the needle is passed in one direction only it does not cause the extensive trauma that TESA produces and as it acquires a large piece of testicular tissue it offers the same SRR as cTESE without the need for an incision.
Single Seminiferous Tubule (SST) mapping [Citation3,Citation4]. The scrotum is opened and the testis is exposed. The tunica is punctured at an avascular area with a 22-G needle. The puncture hole is stretched with the prong of a micro-forceps, so that a loop of seminiferous tubule pops out. This is grasped with a micro-forceps and the tubule is pulled out of the testis and inspected under the operating microscope. If it is thin and gelatinous then no more tubule is extracted from that location; it is cut and the small piece is transferred to medium. If the tubule looks healthy then more of it is pulled out until a sizeable length is delivered. It is then cut and transferred into medium. This allows a large piece of testicular tissue to be harvested without a cut or suture on the tunica. As the procedure is atraumatic it can be repeated all over the testis. Usually 20–30 (depending on the size of the testis) such biopsies are taken from all over the testis, thus mapping it very comprehensively. All tissue is immediately evaluated in the IVF laboratory for sperm; if sperm are found then more tissue is taken from that location. The SST technique allows for extensive sampling of the testis in the least traumatic manner. As the biopsies are from the surface, and under vision, it is less traumatic than even a percutaneous needle biopsy, and is far less traumatic than micro-TESE as the tunical vessels are not damaged and there is no parenchymal dissection.
SST sampling will not be satisfactory if the testis is small and fibrotic, or if the tissue is atrophic and gelatinous. However, in a good-sized testis it can provide enough tissue, with wide sampling equivalent to micro-TESE. It does not sample the depth of the testis and hence would be inferior to micro-TESE in a case where there is very focal spermatogenesis. Whilst it is meant to be performed under an operating microscope, it can be done with operating loupes, thus making it an alternative to micro-TESE in those centres that lack microsurgical facilities.
Micro-TESE. [Citation28] The scrotum is incised and the testis is exposed. The tunica is incised along the entire transverse axis of the testis. The protruding parenchyma is inspected for dilated tubules, which are biopsied and checked for sperm. If no sperm are found then the testis is bivalved and the two cut halves are explored. Blood vessels can be seen radiating from the hilum and the parenchyma is dissected between these vessels. Eventually, the entire parenchyma can be everted over a finger placed on the outer surface of the tunica. This allows the entire testis to be inspected; most of it would be a mixture of atretic and thin tubules. The surgeon looks for tubules that are larger or ‘healthier-looking’ than the neighbouring parenchyma. These are biopsied. However, often the entire parenchyma is uniform (in men with maturation arrest). In such cases, multiple random micro-biopsies are taken from all over. All the biopsies are teased open and checked for sperm. If no sperm are found on one side then the other side is explored (we find sperm on the other side in ∼10% of cases). Careful haemostasis with a micro-bipolar cautery is important. The tunica is closed with 5–0 or 6–0 polypropylene sutures (Prolene®; Ethicon Endo-Surgery, Cincinnati, OH, USA).
Choice of operative procedure
The ideal method is one that retrieves an adequate number of sperm with the least trauma to the testes, and the least discomfort and expense to the patient. Hence, the choice of procedure should be customised to the patient, instead of applying one procedure to all cases, as is done at many centres.
There is no role for epididymal aspiration in NOA, as there is no obstruction and the epididymis is collapsed. In NOA, sperm have to be retrieved from the testis.
As micro-TESE is the most efficient method for retrieving sperm, many centres routinely do micro-TESE for all patients clinically suspected to have NOA. Whilst this is an efficient approach, it does subject many men to an invasive and expensive micro-TESE when sperm may have been found by simpler and less traumatic procedures like nTESE or SST mapping.
Other centres that lack advanced andrological facilities limit themselves to TESA or an open biopsy; if no sperm are found they do not proceed further, or may then refer the patient to another centre for micro-TESE later on. This approach is not optimal as the first procedure (TESA or cTESE) will compromise testicular function to some extent and reduce the chances of success of the subsequent micro-TESE, especially in those men who already have very limited spermatogenesis.
Thus, recognising that in some men with NOA sperm may be retrieved by a simple NAB or SST mapping, whilst others will need micro-TESE, and that the first attempt offers the best chance of sperm retrieval, we have developed an integrated approach: single-session staged sperm retrieval (SSSSR), as described below.
The patient and operating room are prepared for micro-TESE. We start by doing three NABs on one testis. If adequate sperm are found the procedure is over. If no sperm are found then three more NABs are done on the opposite side. If no sperm are found the scrotum is opened and one testis is exposed, and 15–30 SST (depending on testis size) biopsies are taken, thus mapping the entire testicular surface. The samples are checked in the IVF laboratory for sperm (SST mapping may be abandoned if the testis is very small and fibrotic or if only thin gelatinous strands are obtained in the first few SST biopsies). If no sperm are found in the mapping samples then the testis is bi-valved and micro-TESE is done. If no sperm are found then the opposite testis is explored and SST mapping followed by micro-TESE is done. This SSSSR approach gives the best chance of sperm retrieval whilst reducing the need for aggressive procedures.
Sperm retrieval in ejaculatory failure
Whilst sperm retrieval from the epididymis and vas has been reported in men with ejaculatory failure, our preference is for testicular NAB rather than epididymal aspiration for two reasons:
Since there is no obstruction often no sperm are recovered from the epididymis.
Epididymal puncture can damage the ductule and create an obstruction that would hamper future sperm in the ejaculate.
Sperm retrieval in necrozoospermia
If all the sperm in the ejaculate are immotile and dead (on viability testing) then one can still retrieve viable sperm (motile or immotile) from the testis by NAB.
Conclusion
The urologist has an important role to play in operative sperm retrieval in azoospermic men. Whilst many centres use a single method for sperm retrieval in all patients, the best results would be obtained by individualising the procedure to each man’s clinical situation.
The ideal procedure should be highly effective whilst causing the least necessary trauma and discomfort. The first attempt should be the best attempt. A staged approach, during a single session would help avoid more extensive procedures in many patients, whilst giving the best chance of sperm retrieval.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- J.R.KovacK.J.LehmannM.A.FischerA single-center study examining the outcomes of percutaneous epididymal sperm aspiration in the treatment of obstructive azoospermiaUrol Ann620144145
- S.K.GirardiP.SchlegelMESA: review of techniques, preoperative considerations and resultsJ Androl17199659
- R.S.ShahSurgical and non-surgical methods of sperm retrievalM.HansotiaS.DesaiM.PariharAdvanced infertility management2002Jaypee BrothersNew Delhi253258
- R.S.ShahSurgical sperm retrieval: techniques and their indicationsIndian J Urol272011102109
- A.A.DabajaP.N.SchlegelMicrodissection testicular sperm extraction: an updateAsian J Androl1520133539
- D.H.ShinP.J.TurekSperm retrieval techniquesNat Rev Urol102013723730
- Y.DeruyverD.VanderschuerenF.Van der AaOutcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia: a systematic reviewAndrology220142024
- S.C.EstevesClinical management of infertile men with nonobstructive azoospermiaAsian J Androl172015459470
- A.M.BernieD.A.MataR.RamasamyP.N.SchlegelComparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta-analysisFertil Steril104201510991103
- R.FlanniganP.V.BachP.N.SchlegelMicrodissection testicular sperm extractionTransl Androl Urol62017745752
- P.N.SchlegelL.M.SuPhysiological consequences of testicular sperm extractionHum Reprod12199716881692
- M.AmerA.AteyahR.HanyW.ZohdyProspective comparative study between microsurgical and conventional testicular sperm extraction in non-obstructive azoospermia: follow-up by serial ultrasound examinationsHum Reprod152000653656
- C.F.JensenD.A.OhlM.R.HinerM.FodeT.ShahG.D.Smithet alMultiple needle-pass percutaneous testicular sperm aspiration as first-line treatment in azoospermic menAndrology42016257262
- K.EveraertI.De CrooW.KerckhaertP.DekuyperM.DhontJ.Van der Elstet alLong term effects of micro-surgical testicular sperm extraction on androgen status in patients with non-obstructive azoospermiaBMC Urol20200669
- R.RamasamyN.YaganP.N.SchlegelStructural and functional changes to the testis after conventional versus microdissection testicular sperm extractionUrology65200511901194
- G.M.ColpiE.M.ColpiG.PiediferroD.GiacchettaG.GazzanoF.M.Castiglioniet alMicrosurgical TESE versus conventional TESE for ICSI in non-obstructive azoospermia: a randomized controlled studyReprod BioMed Online182009315319
- I.F.GhalayiniM.A.Al-GhazoO.B.HaniR.Al-AzabI.Bani-HaniF.Zayedet alClinical comparison of conventional testicular sperm extraction and microdissection techniques for non-obstructive azoospermiaJ Clin Med Res32011124131
- V.VernaeveH.TournayeJ.SchiettecatteG.VerheyenA.Van SteirteghemP.DevroeySerum inhibin B cannot predict testicular sperm retrieval in patients with non-obstructive azoospermiaHum Reprod172002971976
- I.PlottonA.BrosseB.CuzinH.LejeuneKlinefelter syndrome and TESE-ICSIAnn Endocrinol (Paris)752014118125
- P.J.StahlP.MassonA.MielnikM.B.MareanP.N.SchlegelD.A.PaduchA decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermiaFertil Steril94201017531756
- S.C.EstevesA.AgarwalRe: sperm retrieval rates and intracytoplasmic sperm injection outcomes for men with non-obstructive azoospermia and the health of resulting offspringAsian J Androl162014642
- A.HusseinY.OzgokL.RossP.RaoC.NiederbergerOptimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre studyBJU Int1112013E1104
- K.ShiraishiC.OhmiT.ShimabukuroH.MatsuyamaHuman chorionic gonadotropin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermiaHum Reprod272012331339
- J.E.ReifsnyderR.RamasamyJ.HusseiniP.N.SchlegelRole of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermiaJ Urol1882012532536
- I.CraftM.TsirigotisSimplified recovery, preparation and cryopreservation of testicular spermHum Reprod10199516231627
- E.PrithivirajS.SureshM.ManivannanS.PrakashImpact of sperm retrieval [corrected] on testis and epididymis: an experimental study using Wistar albino ratsSyst Biol Reprod Med592013261269
- M.Gil-SalomJ.RomeroY.MinguezM.D.MoleroJ.RemohlA.PellicerTesticular sperm extraction and intracytoplasmic sperm injection: a chance of fertility in nonobstructive azoospermiaJ Urol160199820632067
- P.N.SchlegelTesticular sperm extraction: microdissection improves sperm yield with minimal tissue excisionHum Reprod141999131135