Abstract
Objective: To summarise the current state of research into spermatogonial stem cell (SSC) therapies with a focus on future directions, as SSCs show promise as a source for preserving or initiating fertility in otherwise infertile men.
Materials and methods: We performed a search for publications addressing spermatogonial stem cell transplantation in the treatment of male infertility. The search engines PubMed and Google Scholar were used from 1990 to 2017. Search terms were relevant for spermatogonial stem cell therapies. Titles of publications were screened for relevance; abstracts were read, if related and full papers were reviewed for directly pertinent original research.
Results: In all, 58 papers were found to be relevant to this review, and were included in appropriate subheadings. This review discusses the various techniques that SSCs are being investigated to treat forms of male infertility.
Conclusions: Evidence does not yet support clinical application of SSCs in humans. However, significant progress in the in vitro and in vivo development of SSCs, including differentiation into functional germ cells, gives reason for cautious optimism for future research.
Abbreviations:
- ART
- assisted reproductive technologies
- Bcl6b
- B-Cell CLL/Lymphoma 6B
- BMP4
- bone morphogenetic protein 4
- CD(24)(34)
- cluster of differentiation (24)(34)
- c-Kit
- KIT Proto-oncogene receptor tyrosine kinase
- FGF2
- Fibroblast growth factor 2
- FISH
- fluorescence in situ hybridisation
- GDNF
- glial cell line-derived neurotrophic factor
- ICSI
- intracytoplasmic sperm injection
- ID4
- inhibitor of differentiation 4
- KS
- Klinefelter syndrome
- PGC
- primordial germ cells
- PLZF
- promyelocytic leukaemia zinc finger
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RA(R)
- retinoic acid (receptor)
- SSC
- spermatogonial stem cell
- SPG
- spermatogonia
- Stra8
- stimulated by RA 8
- ZBTB
- zinc finger and broad complex/Tramtrack/bric-a-brac
Introduction: the unaddressed need in male infertility
Infertility is defined as an inability to achieve pregnancy despite 12 months of unprotected intercourse at regular intervals [Citation1]. This occurs in ∼15% of couples [Citation1]. The prevalence of male factor contribution to infertility is difficult to estimate, probably because of under-reporting. Estimates for male factor-only infertility range from 6.4% to 42.4%, and estimates of male factor contributing to infertility range from 18.8% to 39% [Citation1].
The causes of male infertility include varicoceles, medications, obstruction, and genetic disorders [Citation2]. Treatments currently fall into several categories for the male: relief of obstruction, optimisation of sperm production, and surgical extraction of sperm [Citation3]. Obstruction can be relieved through microsurgical techniques, obviating the need for stem cell therapy. Varicocele repair improves rates of pregnancy with assisted reproductive technologies (ART) for oligospermic and azoospermic men [Citation4]. Although controversial, varicocele repair may even improve semen analysis in selected cases of azoospermic men [Citation5].
For men who are unable to improve their semen analysis adequately for natural conception, ART are available. The most drastic of these is intracytoplasmic sperm injection (ICSI), a micro-manipulation technique wherein a single spermatozoon is inserted into an oocyte [Citation6]. This has allowed for successful pregnancies in cases where male factor infertility has drastically reduced sperm counts below the levels that would be successful with traditional in vitro fertilisation (IVF) [Citation6].
Sperm retrieval techniques include epididymal or testicular microsurgical sperm retrieval [Citation3]. These can obtain sperm for use in ART. However, all these treatments require that the male produces his own sperm, even at dramatically decreased levels. Barring the finding of sperm on effective dissection/exploration, the patient is currently considered unable to produce his own genetic offspring. Stem cell therapy using spermatogonial stem cells (SSCs) is an emerging field that aims to rectify this.
The basic premise of SSC therapy is to induce spermatogenesis from the man’s own non-functioning, poorly functioning, or undifferentiated SSCs. These spermatogonia (SPG) may be supported in vivo within the patient’s testis or in xenograft or other ex vivo culture. The sperm obtained from this could then be used for fertilisation, with or without ART.
SSC therapy has the potential to have wide clinical applications, e.g. in degenerative diseases of the testes, such as Klinefelter syndrome (KS) [Citation7]. In KS, progressive hyalinisation occurs in the testes and SPG lose the ability to replenish themselves, especially at and beyond puberty [Citation7]. This may eventually lead to complete absence of SPG. Although SPG from men with KS may behave differently than SPG from men with a normal karyotype, preserving SPG for differentiation in the future could help address the 30% of men with unsuccessful surgical sperm retrieval [Citation7].
The purpose of the present review is to provide an update on the research in the field of SSCs as it relates to the clinician.
Methods and results
We performed a search for publications addressing SSC transplantation in the treatment of male infertility. The search engines PubMed and Google Scholar were used from 1990 to 2017. The search terms included: ‘spermatogonial stem cell’, ‘spermatogenesis’, ‘stem cell’, ‘in vitro’, ‘xenograft’, ‘autologous transplantation’, ‘allograft’, ‘fertility preservation’, ‘pluripotent’, ‘pluripotency’, and ‘embryonic stem cell’. Papers titles were screened for relevance, and abstracts were read for pertinent papers. Original relevant research articles were included, and review articles were included for new insight, to provide an in-depth reference of a tangential literature, or used to identify complimentary primary research articles. Representative articles were selected in the case of similar publications. Papers publishing incremental modifications to basic science techniques were omitted. Overall, 701 unique records were screened and 58 articles were included in this review. These were comprised of 16 reviews and 42 original research articles. The synthesis of these articles was qualitative in nature and can be found in the corresponding subheadings below. This is represented graphically using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) format in [Citation8].
SSCs: a brief overview
Stem cells are defined as cells with the ability to make copies of themselves indefinitely (self-renewal), and also with the ability to differentiate into other cell types [Citation9]. Totipotent stem cells can differentiate into all cell types including extra-embryonal cells, whilst pluripotent cells can differentiate into every cell in the human body but not extra-embryonal cells [Citation9]. Multipotent cells can differentiate into multiple cell types within one germ layer, and unipotent cells can differentiate into several or only one cell type [Citation10].
SSCs are a type of undifferentiated spermatogenic cell [Citation11]. SSCs have pluripotent potential [Citation12], but frequently progress into progenitor SPG that eventually differentiate into spermatozoa [Citation11,Citation12]. They maintain their own population through self-renewal [Citation11]. An in depth overview of SSC differentiation and renewal can be found in a review by Phillips et al. [Citation12]. Briefly, SSCs are diploid, and are found on the basement membrane of seminiferous tubules. SSCs undergo a mitotic division resulting in two Type A SPG. Type A SPG do not contain heterochromatin in the nucleus, which indicates a less differentiated state. Type Adark SPG are the self-renewal population, and replenish the SSC population. TypeApale SPG go on to transition into Type B SPG and commit to the differentiation process. These Type B SPG progress to become primary spermatocytes, undergo meiosis, and eventually form spermatozoa [Citation12,Citation13].
This complex self-renewal and differentiation process is under regulation by intrinsic and extrinsic factors, and is not fully elucidated [Citation11]. Extrinsically, self-renewal is modulated by glial cell line-derived neurotrophic factor (GDNF). GDNF is secreted by Sertoli cells, and decreased expression leads to loss of SSCs with age in mice [Citation11]. Fibroblast growth factor 2 (FGF2) is also required in culture, although its function may partially overlap with the GDNF pathway [Citation11]. FGF2 and GDNF exert their effect through the mitogen-activated protein kinase (MAPK)1/3 [synonymous with extracellular signal-regulated kinase (ERK)1/2] signalling inducing G1 to S transition [Citation14]. GDNF stimulation of GDNF family receptor α1 (GFRα1) also co-signals activation of RET (REarranged during Transfection) receptor leading to upregulation of Src family kinase (SFK) activating several transcription factors [B-cell CLL/lymphoma 6B (Bcl6b), inhibitor of differentiation 4 (ID4), ETS variant 5 (Etv5), LIM homeobox (1Lhx1)] [Citation15]. In the presence of GNDF, IGF-1 produced by Leydig cells, promotes SSC proliferation via stimulating G2/M progression [Citation16,Citation17]. Further contributors to extrinsic signals for self-renewal include colony stimulating factor 1 (CSF1) and WNT family member 5A (WNT5A) [Citation11]. Intrinsically, some self-renewal factors are induced by GDNF. Bcl6b is a transcript induced by GDNF that is of importance [Citation11]. GDNF-independent factors include promyelocytic leukaemia zinc finger (PLZF), a zinc-finger protein [Citation11]. Octamer-4 (OCT4) and zinc finger and broad complex/Tramtrack/bric-a-brac (ZBTB) promote self-renewal [Citation18].
Differentiation of SSCs require a different set of pathways. Retinoic acid (RA) is integral to inducing meiosis via the RA receptor (RAR) [Citation11]. This induces differentiation, and down-regulates factors such as PLZF [Citation11]. The receptor KIT proto-oncogene receptor tyrosine kinase (c-Kit) can be bound by stem cell factor (SCF) secreted by Sertoli cells, which initiates a signalling cascade for differentiation [Citation11]. Bone morphogenetic protein 4 (BMP4) works in synergy with RA signalling, whilst the antagonist to BMP4, Noggin, functions to prevent RA-induced expression of c-Kit and stimulated by RA 8 (Stra8) [Citation19]. Intrinsic factors involved in differentiation include neurogenin 3 (Ngn3), whose downstream effect is not yet known [Citation11].
SSCs interact with their surrounding environment in the seminiferous compartment, but also receive signals from the testicular interstitium that may influence spermatogenic function [Citation20]. The androgen receptor protein is expressed by foetal gonocytes and is thought to respond to Leydig cell secreted testosterone to suppress proliferation [Citation20]. Testicular macrophages may influence SSCs through direct or indirect signalling pathways [Citation20]. Peritubular myoid cells may also play a contributory role, possibly through androgen receptor activation and GDNF signalling to SSCs [Citation20].
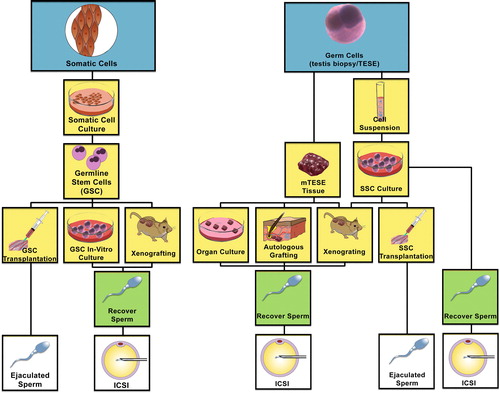
This self-renewal and differentiation capacity allows the organism to produce sperm as long as there are functioning SSCs. Methods to encourage SSC function outside of the normal host are being researched ().
Fig. 2 Flow chart describing the various directions of investigation and therapeutic translation of stem cell differentiation to ultimately become spermatozoa amenable to achieving pregnancy. (Left) Somatic cells may be de-differentiated into induced pluripotent stem cells (iPSCs), and then re-programmed to differentiate through germ cell lineage via transplantation into the testis seminiferous tubules, xenografting or germline stem cells in culture. (Right) SSCs may be harvested from the testis and kept as a tissue biopsy or processed into a single cell suspension. The tissue biopsy may be treated as an organ culture, autologous graft or xenograft to proliferate and differentiate SSCs to spermatozoa. Cell suspensions may be grown in culture and xenografted, autotransplanted into the testis seminiferous tubules, or differentiated in culture to harvest spermatozoa. (m) TESE, (microdissection) testicular sperm extraction.
In vitro growth of SSCs to sperm
As discussed above, SSCs rely on signalling from a surrounding microenvironment or ‘niche’ within the testis. These signals help to balance the self-renewal and differentiation of SSCs to ensure that adequate sperm is created, whilst maintaining the underlying SSC population [Citation12]. Recreating the necessary signals in vitro may be difficult. However, for many years mouse SSCs have been able to be grown in vitro for short periods [Citation21], and more recently functional mouse SSC lines have been maintained in vitro with successful fertilisation [Citation22]. Research is ongoing into methods to simulate the SSC niche in vitro and to optimise SSC growth, including through use of nanofibre scaffolds and culture environment optimisation [Citation23,Citation24]. The original culture media from Nagano et al. [Citation21] was a standard medium containing Dulbecco modified Eagle medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin. The niche may have been partially maintained as donor testis cells were grown together, without isolating SSCs specifically. Supplementation of the media has since included addition of GDNF, FGF2, lipid mixtures, and co-culture with cells such as testicular stroma [Citation24].
Successful propagation of human SSCs has also been performed in vitro, often by culturing testis cells all together rather than isolating SSCs. Testis material from human orchidectomies for prostate cancer were cultured in media over multiple passages, and the presence of SSCs were confirmed at 28 weeks by fluorescence in situ hybridisation (FISH) [Citation25]. PLZF was used as the marker for FISH. Total RNA was also assayed with PCR to ensure expression of spermatogonial genes throughout [Citation25]. This was also repeated with prepubertal SSCs, including transplantation of the cells into mice where the cells remained present for 8 weeks [Citation26]. Function of these SSCs was not assayed, although they continued to display the appropriate markers at the endpoint.
Functional success has meanwhile been achieved for animal SSCs. Testis tissue from neonatal mice, which would not contain sperm but simply primitive SPG, was cultured in vitro for 2 months and sperm were observed [Citation27]. Green fluorescent protein (GFP)-hybrid proteins specific for meiosis were used to identify spermatogenesis in vitro [Citation27]. These sperm were then used for micro-insemination and live offspring were obtained, who themselves were fertile [Citation27].
Success of spermatogonial culture has been found in vitro for larger animals as well. Rat spermatogonial cells were differentiated into cells that had the properties of spermatids under morphological examination after staining [Citation28]. A similar study in goats was able to produce blastocytes for 7 days [Citation29].
However, an ongoing challenge in SSC culture is sorting and isolating SSCs. Numerous identification techniques for SSCs are used, including morphology, surface markers, and functional identification [Citation30]. Morphology is assessed via microscopy, evaluating cells for location and structure. SSCs are located at the basal membrane, and have an ovoid shape. However, types of immature SPG cannot be differentiated using morphology alone, and require other adjunct markers [Citation30].
Currently there is no single unique marker for SSCs, so multiple markers together must be used to confirm their isolation. SSCs are surface marker positive for integrins α6 and β1, cadherin 1 (CDH1), GFRα1, ID4, ZBTB16 (synonymous with PLZF), ret proto-oncogene (RET), thymus cell antigen 1 (Thy-1), and cluster of differentiation 24 (CD24), whilst negative for major histocompatibility complex class I (MHC-I), c-Kit, and CD34, and stimulated by RA (Stra8) expression amongst others [Citation12]. This helps to differentiate SSCs from the surrounding testicular cells [Citation30]. Future research on identifying unique markers would simplify this process [Citation30].
Another way to identify SSCs is a functional assay. SSCs are known to propagate in recipient testes, and their presence can be approximated by evaluated recipient testicles after transplantation. However, this method takes significant resources including time [Citation30].
Concerns about genetic and epigenetic stability in preserved and cultured specimens have been raised. Instability could theoretically lead to carcinogenesis. A thorough investigation of the epigenetic programming of stem cells in vivo is necessary prior to clinical fertilisation with any sperm developed from stem cells in vitro [Citation12].
Cryopreservation does not seem to affect stability, with fertile offspring produced from mouse SSCs from testis tissue preserved for >14 years and re-implanted into nude mice [Citation31]. No major chromosomal abnormalities or DNA-methylation differences, which would indicate epigenetic change, were observed in offspring compared to wild-type [Citation31].
In vitro culture is another area of concern for genetic instability. SSCs cultured for >2 years demonstrated stability of euploid karyotype, with fertile offspring [Citation32]. This showed good stability relative to other stem cell types, such as embryonal stem cells. However, telomeres were found to shorten, indicating that SSCs may not be able to proliferate indefinitely [Citation32]. However, other studies have detected genetic changes. In the ageing of SSCs in vitro, longer culture time was found to decrease DNA expression for genes important in SSC function [Citation33]. These included decreases in the expression of Bcl6 and Lhx1, which are important for self-renewal, and also decreased expression of the Thy-1 surface marker [Citation33]. These changes occurred without obvious morphological changes [Citation33].
The risk of oncogenesis from contamination must also be considered, as future applications for oncology patients are being considered. Testis allografts from T-cell leukaemic rats caused leukaemia in recipients when as few as 20 cells were present [Citation34]. This raises serious concerns about cancer relapse in humans. Relapse from cancer cell contamination is a concern for auto-transplantation of SSCs, especially if these were collected before cancer treatments. Research is ongoing, with a pilot study already finding success in eliminating acute lymphoid leukaemia cells from testis culture [Citation35]. PCR was used for detection of cancer cells, which died after culture for 26 days under testicle cell culture, whilst the testicle cells were able to proliferate [Citation35].
Grafting of stem cells by xenotransplantation
Xenotransplantation, where cells from one species are transplanted into the microenvironment of another species, remains a useful tool in the growth of SSCs. The first documented occurrence of SSC xenografting from humans occurred in 2002, when preparations from testis biopsies of six infertile men were injected into the rete testis of nude mice [Citation36]. These were found to survive for up to 6 months in the host testes, albeit in severely decreased numbers, and without assaying their function [Citation36]. This has been successfully replicated with rat, rabbit, and baboon testis tissue, which has been successfully induced to propagate when transplanted into the rete testis of nude mice [Citation31]. In addition, pig and goat testicular tissue has been able to undergo spermatogenesis in mouse xenografts [Citation37].
Many examples of xenotransplantation, as previously mentioned, also make use of in vitro techniques [Citation26,Citation25]. Often this involves temporarily growing SSC samples in vitro before injection into the host. This method has been performed with human samples. During the diagnosis of maturation arrest in humans, 16 human donor samples from eight patients were cultured in vitro, then transplanted into the rete testis of immune-deficient mice who had been rendered infertile by busulfan treatment [Citation38]. These cells proliferated in the host along the basement membrane, although no sperm were isolated. This suggested that complete differentiation may require human signalling factors [Citation38].
Another hybrid technique is the in vitro transplantation technique, where donor SSCs are cultured in vitro, then injected into host testis, and finally a donor-host mix of tissue fragments is then induced into spermatogenesis in vitro culture once again [Citation39]. This allows for ease of observation of cells compared to pure in vivo xenografting [Citation39].
Xenografting has been found to accelerate development of human infant SSCs. In a separate study, testicular fragments from a 3-month-old human infant were transplanted into the empty scrotum of castrated mice, and growth of the fragments and spermatogenesis, including the presence of spermatocytes, were observed at 1 year [Citation40]. This was increased from the expected speed of development in humans of about 8–10 years of age for spermatocyte development [Citation40].
There have been limited results in the xenografting of adult testis tissue. Although germ cells can survive in xenografting, no complete spermatogenesis was observed in a study where adult testis tissue was xenografted into immune-deficient mice [Citation41]. Many samples in this study did not have functional spermatogenesis to begin with; however, it is worth noting that there was no recovery of spermatogenic function for those samples [Citation41].
One of many remaining questions is the optimal location for SSC injection in the host. One small study in sheep found that insertion of SSCs into the extra-testicular rete testis, with or without ultrasonographic guidance or reflection of the epididymis, provided the best success rate and seminiferous tubule filling compared to intratesticular rete testis techniques [Citation42]. However, there are other conflicting reports. The intratesticular rete testis was found to be superior for testes with a larger volume-to-surface ratio than in mice [Citation43]. In the only human study, a cadaveric study showed that the most efficient location of injection of contrast substance was in the rete testis, near the caput of the epididymis [Citation44].
Grafting of stem cells by allotransplantation/autotransplantation
Transplantation of SSCs into a member of the same species or back into the original host is a useful technique for preserving SSCs and sperm outside the donor. This has numerous theoretical applications, including restoring fertility after fertility-ablative treatments in pre-adolescents [Citation45].
Autologous transplantation has been performed in rodents. Mice had Sertoli cells and spermatogonial cells isolated and transplanted into their own contralateral testicle after irradiation, with morphological changes consistent with spermatogenesis viewed at 8 weeks compared to the control testicle [Citation46]. Interestingly, SSCs injected into the tubule lumen are able to transmigrate to the basement membrane via attachment to the Sertoli cells using laminin receptor integrin α6 and β1, chemokine C-X-C motif receptor 4 (CXCR4), and RAS-related C3 botulinum substrate 1 (RAC1) [Citation47,Citation48]. Furthermore, SSC transmigration occurs preferentially to seminiferous tubules juxtaposed to interstitial regions rich with Leydig cells, macrophages and capillaries [Citation49].
As techniques of in vitro and combined in vitro and in vivo approaches for grafting improve, autologous transplantation of SSCs has also taken place in larger animals. Bovine autologous transplant was successful in restoring morphology to irradiated testes [Citation50]. Success was reported in growing testicular volume by autologous transplantation of germ cells into the rete testis of monkeys who were rendered infertile [Citation51]. Small numbers of sperm were seen, and testicular volume showed an increase compared to the contralateral control [Citation51]. However, in another study of four prepubertal and two pubertal monkeys also rendered infertile and transplanted with autologous testicular cells, only one prepubertal monkey showed increased testicular volume in the transplanted testicle [Citation52]. The next year in 2012, Hermann et al. [Citation53] produced functional sperm after autologous and allogenic transplantation in rhesus monkeys that were rendered infertile.
For humans, a paper from Manchester in 2000 made reference to an ongoing clinical trial where 11 men had testicular tissue harvested prior to chemotherapy, and seven had reinjection of this tissue into the rete testes after treatment was completed [Citation54]. Unfortunately, further details have not been published to date.
Temporary grafting of SPG for young males undergoing fertility-ablative treatment
Grafting spermatogonial stem cells by autotransplantation may be a beneficial infertility treatment in younger males who are to undergo fertility-ablative treatments such as chemotherapy or radiotherapy. These treatments have the unintended consequence of affecting the spermatogenic system because of its high proliferative activity [Citation55]. Infertility has a major negative effect on quality of life in cancer survivors [Citation56]. Sperm cryopreservation is an option for adult patients with cancer who will undergo treatments that may interfere with fertility [Citation57]. For younger males who do not yet produce sperm, this is not an option. Patients receiving immunosuppression for non-cancer illnesses are also at risk of reduced fertility and would also benefit from another option for fertility preservation [Citation45].
Despite the lack of mature sperm, SSCs are still present in the younger male population. Harvesting these SSCs is possible, with efforts being made to develop a protocol for future differentiation into sperm later in life when the cancer survivor wishes fertility [Citation58]. Slow-freezing protocols are available to preserve human testicular tissue over the longer term, and competing protocols such as vitrification have also been developed [Citation59]. Determining how to then safely re-inject these SSCs and induce spermatogenesis later in life would be beneficial and is currently under investigation.
To date, no functional SPG have been obtained from SSCs from prepubertal humans. Much of the animal research pertinent to this area is touched upon in the above sections. However, testicular tissue has been isolated from patients aged 10 and 11 years who underwent bone marrow depletion, and transplanted in xenograft fashion onto the back of nude mice for 4–9 months [Citation60]. Morphology was examined, and SPG were found to survive although spermatogenesis was not observed [Citation60]. As the animal models for transplantation improve, further advances are expected in this area.
Inducing pluripotent stem cells into SSCs
For patients who do not have SSCs of their own, research is ongoing to induce pluripotent stem cells to differentiate into SSCs. As mentioned previously, pluripotent stem cells can differentiate into all human cells, which should theoretically include SSCs.
Pluripotent stem cells can be obtained through multiple mechanisms. Several often-studied mechanisms are harvesting of embryonal stem cells, reprogramming adult somatic cells to make induced pluripotent stem cells, or from somatic cell nuclear transfer (SCNT) where a nucleus is inserted into an oocyte [Citation61]. In humans, of course, the initial embryonic development is guided by centrioles from male-derived germ cells, limiting the potential success of using nuclear transfer alone (or male germ cells that have not yet initiated tail development.) The traditional pathway suggests that pluripotent stem cells must be differentiated into primordial germ cells (PGCs) prior to SSCs [Citation61]. A review of the mechanisms for this differentiation can be found by Nikolic et al. [Citation62].
Several studies have been able to differentiate embryonal stem cells into male germ cells. In one striking study, mouse SSCs were developed in vitro from embryonic cells, and differentiated into sperm-like structures [Citation63]. These structures were then successfully used to fertilise a wild-type oocyte, with resultant live birth [Citation63]. However, the success rate of embryogenesis was low, severe phenotypic abnormalities were noted, and the offspring died prematurely [Citation63]. Human embryonal cord-derived perivascular cells have also been successfully cultured in vitro in an environment designed to simulate the testis, and were induced to differentiate into cells that resembled Sertoli cells and haploid spermatid-like cells [Citation64]. Several other similar studies met with varying degrees of success, and can be found summarised in a review by Hou et al. [Citation65]. Induced pluripotent stem cells have also had some success in differentiation into SSCs but carcinogenesis concerns have precluded clinical use in humans to date [Citation65].
Research is also ongoing into direct differentiation of pluripotent stem cells into SSCs, bypassing the PGC differentiation step. Human embryonal stem cells and human pluripotent stem cells have been induced in vitro into haploid cells that resemble spermatid-like cells based on molecular markers, although not functional assays [Citation66]. This was performed without an explicit PGC differentiation protocol [Citation66]. These differentiated haploid cells could then theoretically be transplanted back into the donor testis if the microenvironment would support them, or continue differentiation into functional sperm for ART [Citation66]. Further functional testing is required.
In summary, pluripotent stem cell research into induction into SSCs is ongoing with multiple cell types, either through differentiation first into PGCs or directly into SSCs, with much further functional testing required.
Conclusion
SSCs show promise for application for future clinical practice. Research in vitro and in animal or human/animal models of auto/allo/xenografting have shown some functional success, including production of fertile offspring after culture in animals. There is cause for cautious optimism, with significant barriers including concern about carcinogenesis and genetic/epigenetic changes in offspring remaining to be fully addressed and translation into humans.
Acknowledgements
Medical illustrator Vanessa Dudley.
Source of funding
Ryan Flannigan: American Urology Association New York Section E. Darracott Vaughan, Jr, MD, Research Scholar Award.
Conflict of interest
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- B.R.WintersT.J.WalshThe epidemiology of male infertilityUrol Clin North Am412014195204
- S.QuallichExamining male infertilityUrol Nurs262006277288
- M.GoldsteinC.TanrikutMicrosurgical management of male infertilityNat Clin Pract Urol32006381391
- E.W.KirbyL.E.WienerS.RajanahallyK.CrowellR.M.CowardUndergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: a systematic review and meta-analysisFertil Steril106201613381343
- A.MehtaM.GoldsteinVaricocele repair for nonobstructive azoospermiaCurr Opin Urol222012507512
- G.D.PalermoQ.V.NeriT.TakeuchiZ.RosenwaksICSI: where we have been and where we are goingSemin Reprod Med272009191201
- L.AksglaedeA.JuulTesticular function and fertility in men with Klinefelter syndrome: a reviewEur J Endocrinol1682013R67R76
- D.MoherA.LiberatiJ.TetzlaffD.G.AltmanPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementAnn Intern Med151264–92009w64
- S.MeachemV.von SchönfeldtS.SchlattSpermatogonia: stem cells with a great perspectiveReproduction1212001825834
- M.A.SoebadiL.MorisF.CastiglioneE.WeyneM.AlbersenAdvances in stem cell research for the treatment of male sexual dysfunctionsCurr Opin Urol262016129139
- X.X.MeiJ.WangJ.WuExtrinsic and intrinsic factors controlling spermatogonial stem cell self-renewal and differentiationAsian J Androl172015347354
- B.T.PhillipsK.GasseiK.E.OrwigSpermatogonial stem cell regulation and spermatogenesisPhilos Trans R Soc Lond, B, Biol Sci365201016631678
- S.Z.JanT.L.VormerA.JongejanM.D.RolingS.J.SilberD.G.de Rooijet alUnraveling transcriptome dynamics in human spermatogenesisDevelopment144201736593673
- H.KubotaM.R.AvarbockR.L.BrinsterGrowth factors essential for self-renewal and expansion of mouse spermatogonial stem cellsProc Natl Acad Sci USA10120041648916494
- H.SariolaM.SaarmaNovel functions and signalling pathways for GDNFJ Cell Sci116200338553862
- Y.H.HuangC.C.ChinH.N.HoC.K.ChouC.N.ShenH.C.Kuoet alPluripotency of mouse spermatogonial stem cells maintained by IGF-1-dependent pathwayFASEB J23200920762087
- S.WangX.WangY.WuC.HanIGF-1R signaling is essential for the proliferation of cultured mouse spermatogonial stem cells by promoting the G2/M progression of the cell cycleStem Cells Dev242015471483
- C.T.DannA.L.AlvaradoL.A.MolyneuxB.S.DenardD.L.GarbersM.H.PorteusSpermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiationStem Cells26200829282937
- Y.YangY.FengX.FengS.LiaoX.WangH.Ganet alBMP4 cooperates with retinoic acid to induce the expression of differentiation markers in cultured mouse spermatogoniaStem Cells Int20162016953619210.1155/2016/9536192
- S.J.PotterT.DeFalcoRole of the testis interstitial compartment in spermatogonial stem cell functionReproduction1532017R151R162
- M.NaganoB.Y.RyuC.J.BrinsterM.R.AvarbockR.L.BrinsterMaintenance of mouse male germ line stem cells in vitroBiol Reprod68200322072214
- Z.YuanR.HouJ.WuGeneration of mice by transplantation of an adult spermatogonial cell line after cryopreservationCell Prolif422009123131
- A.ShamsN.EslahiM.MovahedinF.IzadyarH.AsgariM.KorujiFuture of spermatogonial stem cell culture: application of nanofiber scaffoldsCurr Stem Cell Res Ther12201754455310.2174/1574888X12666170623095457
- A.R.HelselM.J.OatleyJ.M.OatleyGlycolysis-optimized conditions enhance maintenance of regenerative integrity in mouse spermatogonial stem cells during long-term cultureStem Cell Rep8201714301441
- H.Sadri-ArdekaniS.C.MizrakS.K.van DaalenC.M.KorverH.L.Roepers-GajadienM.Korujiet alPropagation of human spermatogonial stem cells in vitroJAMA302200921272134
- H.Sadri-ArdekaniM.A.AkhondiF.van der VeenS.ReppingA.M.M.van PeltIn vitro propagation of human prepubertal spermatogonial stem cellsJAMA305201124162418
- T.SatoK.KatagiriA.GohbaraK.InoueN.OgonukiA.Oguraet alIn vitro production of functional sperm in cultured neonatal mouse testesNature4712011504507
- A.RedaM.HouT.WintonR.ChapinO.SöderJ.StukenborgIn vitro differentiation of rat spermatogonia into round spermatids in tissue cultureMol Hum Reprod22201660161210.1093/molehr/gaw047
- S.DengX.WangZ.WangS.ChenY.WangX.Haoet alIn vitro production of functional haploid sperm cells from male germ cells of Saanen dairy goatTheriogenology902017120128
- R.ZhangJ.SunK.ZouAdvances in isolation methods for spermatogonial stem cellsStem Cell Rev1220161525
- X.WuS.M.GoodyearL.K.AbramowitzM.S.BartolomeiJ.W.TobiasM.R.Avarbocket alFertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 yearsHum Reprod27201212491259
- M.Kanatsu-ShinoharaN.OgonukiT.IwanoJ.LeeY.KazukiK.Inoueet alGenetic and epigenetic properties of mouse male germline stem cells during long-term cultureDevelopment132200541554163
- J.A.SchmidtL.K.AbramowitzH.KubotaX.WuZ.NiuM.R.Avarbocket alIn vivo and in vitro aging is detrimental to mouse spermatogonial stem cell functionBiol Reprod842011698706
- K.JahnukainenM.HouC.PetersenB.SetchellO.SöderIntratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemiaCancer Res612001706710
- H.Sadri-ArdekaniC.H.HomburgT.M.M.van CapelH.van den BergF.van der VeenC.E.van der Schootet alEliminating acute lymphoblastic leukemia cells from human testicular cell cultures: a pilot studyFertil Steril10120141072-8.e1
- M.NaganoP.PatrizioR.L.BrinsterLong-term survival of human spermatogonial stem cells in mouse testesFertil Steril78200212251233
- A.HonaramoozA.SnedakerM.BoianiH.SchölerI.DobrinskiS.SchlattSperm from neonatal mammalian testes grafted in miceNature4182002778781
- T.MirzapourM.MovahedinM.KorujiM.NowrooziXenotransplantation assessment: morphometric study of human spermatogonial stem cells in recipient mouse testesAndrologia472015626633
- T.SatoK.KatagiriY.KubotaT.OgawaIn vitro sperm production from mouse spermatogonial stem cell lines using an organ culture methodNat Protoc8201320982104
- Y.SatoS.NozawaM.YoshiikeM.AraiC.SasakiT.IwamotoXenografting of testicular tissue from an infant human donor results in accelerated testicular maturationHum Reprod25201011131122
- S.SchlattA.HonaramoozJ.EhmckeP.J.GoebellH.RubbenR.Dhiret alLimited survival of adult human testicular tissue as ectopic xenograftHum Reprod212006384389
- J.R.Rodriguez-SosaH.DobsonA.HahnelIsolation and transplantation of spermatogonia in sheepTheriogenology66200620912103
- S.SchlattG.RosiepenG.F.WeinbauerC.RolfP.F.BrookE.NieschlagGerm cell transfer into rat, bovine, monkey and human testesHum Reprod141999144150
- L.NingJ.MengE.GoossensT.LahoutteM.MarichalH.TournayeIn search of an efficient injection technique for future clinical application of spermatogonial stem cell transplantation: infusion of contrast dyes in isolated cadaveric human testesFertil Steril9820121443-8.e1
- E.K.JohnsonC.FinlaysonE.E.RowellY.GosiengfiaoM.E.PavoneB.Lockartet alFertility preservation for pediatric patients: current state and future possibilitiesJ Urol1982017186194
- M.KorujiM.MovahedinS.J.MowlaH.GourabiS.Pour-BeiranvandArfaee A.JabbariAutologous transplantation of adult mice spermatogonial stem cells into gamma irradiated testesCell J1420128289
- M.Kanatsu-ShinoharaM.TakehashiS.TakashimaJ.LeeH.MorimotoS.Chumaet alHoming of mouse spermatogonial stem cells to germline niche depends on beta1-integrinCell Stem Cell32008533542
- S.TakashimaM.Kanatsu-ShinoharaT.TanakaM.TakehashiH.MorimotoT.ShinoharaRac mediates mouse spermatogonial stem cell homing to germline niches by regulating transmigration through the blood-testis barrierCell Stem Cell92011463475
- H.F.do NascimentoA.L.DrumondL.R.de FrancaH.Chiarini-GarciaSpermatogonial morphology, kinetics and niches in hamsters exposed to short- and long-photoperiodInt J Androl322009486497
- F.IzadyarK.Den OudenT.A.StoutJ.StoutJ.CoretD.P.Lankveldet alAutologous and homologous transplantation of bovine spermatogonial stem cellsReproduction1262003765774
- S.SchlattL.FoppianiC.RolfG.F.WeinbauerE.NieschlagGerm cell transplantation into X-irradiated monkey testesHum Reprod1720025562
- K.JahnukainenJ.EhmckeM.A.QuaderM.Saiful HuqM.W.EpperlyS.Hergenrotheret alTesticular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantationHum Reprod26201119451954
- B.P.HermannM.SukhwaniF.WinklerJ.N.PascarellaK.A.PetersY.Shenget alSpermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional spermCell Stem Cell112012715726
- J.A.RadfordIs prevention of sterility possible in men?Ann Oncol11Suppl. 32000173174
- K.E.OrwigS.SchlattCryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertilityJ Natl Cancer Inst Monographs3420055156
- J.YiM.A.KimJ.SangWorries of childhood cancer survivors in young adulthoodEur J Oncol Nurs212016113119
- A.AgarwalS.S.R.AllamaneniDisruption of spermatogenesis by the cancer disease processJ Natl Cancer Inst Monographs342005912
- O.HovattaCryopreservation of testicular tissue in young cancer patientsHum Reprod Update72001378383
- Y.BaertD.Van SaenP.HaentjensP.In't VeldH.TournayeE.GoossensWhat is the best cryopreservation protocol for human testicular tissue banking?Hum Reprod28201318161826
- E.GoossensM.GeensG.De BlockH.TournayeSpermatogonial survival in long-term human prepubertal xenograftsFertil Steril90200820192022
- R.VassenaC.EguizabalB.HeindryckxK.SermonC.SimonA.M.van Peltet alStem cells in reproductive medicine: ready for the patient?Hum Reprod30201520142021
- A.NikolicV.VolarevicL.ArmstrongM.LakoM.StojkovicPrimordial germ cells: current knowledge and perspectivesStem Cells Int20162016174107210.1155/2016/1741072
- K.NayerniaJ.NolteH.W.MichelmannJ.H.LeeK.RathsackN.Drusenheimeret alIn vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring miceDev Cell112006125132
- E.ShlushL.MaghenS.SwansonS.KenigsbergS.MoskovtsevT.Barrettoet alIn vitro generation of Sertoli-like and haploid spermatid-like cells from human umbilical cord perivascular cellsStem Cell Res Ther820173710.1186/s13287-017-0491-8
- J.HouS.YangH.YangY.LiuY.LiuY.Haiet alGeneration of male differentiated germ cells from various types of stem cellsReproduction1472014R179R188
- C.A.EasleyB.T.PhillipsM.M.McGuireJ.M.BarringerH.ValliB.P.Hermannet alDirect differentiation of human pluripotent stem cells into haploid spermatogenic cellsCell Rep22012440446