Abstract
Objective: To assess the role of differentially expressed proteins as a resource for potential biomarker identification of infertility, as male infertility is of rising concern in reproductive medicine and evidence pertaining to its aetiology at a molecular level particularly proteomic as spermatozoa lack transcription and translation. Proteomics is considered as a major field in molecular biology to validate the target proteins in a pathophysiological state. Differential expression analysis of sperm proteins in infertile men and bioinformatics analysis offer information about their involvement in biological pathways.
Materials and methods: Literature search was performed on PubMed, Medline, and Science Direct databases using the keywords ‘sperm proteomics’ and ‘male infertility’. We also reviewed the relevant cross references of retrieved articles and included them in the review process. Articles written in any language other than English were excluded.
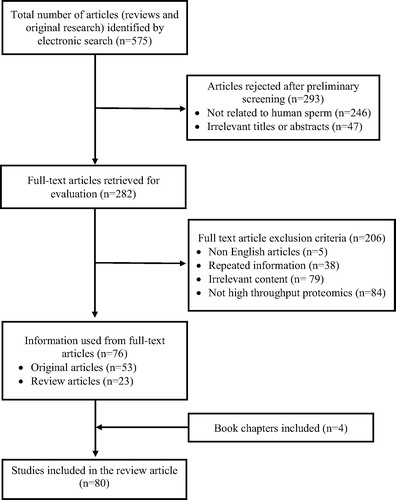
Results: Of 575 articles identified, preliminary screening for relevant studies eliminated 293 articles. At the next level of selection, from 282 studies only 80 articles related to male infertility condition met the selection criteria and were included in this review.
Conclusion: In this molecular era, sperm proteomics has created a platform for enhanced understanding of male reproductive physiology as a potential tool for identification of novel protein biomarkers related to sperm function in infertile men. Therefore, it is believed that proteomic biomarkers can overcome the gaps in information from conventional semen analysis that are of limited clinical utility.
Introduction
Currently, infertility is one of the most addressed issues related to male reproductive dysfunction. Amongst the 9% of the world’s infertility cases, ∼20% is contributed by the male population alone [Citation1]. There are multiple factors that govern and regulate male factor infertility, although most of these cases remain idiopathic. Andrology laboratories rely mainly on semen analysis to evaluate male infertility in patients with poor semen quality. Conventional tests such as basic semen analysis to determine sperm concentration, motility, vitality, and morphology are used for diagnosing male infertility based on reference values established by the WHO [Citation2]. However, advanced laboratory tests such as quantification of reactive oxygen species (ROS)1 and antioxidants in semen by chemiluminescence assay [Citation3], oxidation–reduction potential in semen by Male Infertility Oxidative System (MiOXSYS®; Aytu BioScience Inc., Englewood, CO USA) [Citation4], and sperm DNA fragmentation assessment by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay [Citation5], are clinically used to identify the specific cause of infertility for further utilisation in assisted reproductive technology. However, the aetiological changes at the subcellular level of the spermatozoa remain unknown.
New generation techniques, such as proteomics, are poised to help researchers identify the molecular aspects of spermatozoa that are affected in infertility conditions. The majority of protein biomolecules are involved in cell signalling pathways. Generally, spermatozoa are transcriptionally and translationally silent, so they depend on their proteins to carry out their biological functions [Citation6]. Proteomics is an emerging tool that could potentially help identify protein alterations in spermatozoa and seminal plasma of male infertile patients [Citation7]. Differential expression of sperm proteins in fertility compromised patients is an indicator of defective spermatogenesis, motility, capacitation, hyperactivation, acrosome reaction, and fertilisation processes at a molecular level.
Aberrant expression of proteins in spermatozoa of men causes changes in physiological functions due to post-translational modifications, such as phosphorylation, glycosylation, proteolytic cleavage, and mutations [Citation8,Citation9]. Therefore, it is important to understand the changes in the proteins and cellular pathways affected in infertile patients for better diagnosis in a clinical perspective.
Materials and methods
An extensive search of studies published until October 2017 was performed using PubMed, MedLine, and Science Direct databases. The search was limited to full articles published in the English language and studies on human semen were only included. ‘Sperm proteomics’ and ‘male infertility’ were the two primary keywords used to retrieve articles from different databases. Combination of the following keywords relevant to infertility and proteomics was used to extract the articles: ‘spermatozoa’, ‘sperm proteomics’, ‘varicocele proteomics’, ‘proteome’, ‘oxidative stress and proteomics’, ‘2D-PAGE, mass spectrometry’. Search terms such as ‘azoospermia’, ‘asthenozoospermia’, ‘mitochondrial dysfunction’, ‘testicular cancer and proteomics’ were also used. Cross referencing was also referred to and used in the review process.
Results
Comprehensive literature collection via electronic search resulted in a total of 575 review and original research articles. Preliminary screening resulted in 282 articles that included different proteomic studies from human sperm (). In the subsequent screening, 206 studies were rejected as many (n = 84) were not related to high-throughput proteomics. Finally, 80 full-text articles (review, original research, and book chapters) met the inclusion criteria and were found to be eligible for the review.
Proteomic analysis
Proteins are the functional biomolecules of the cell. Alteration in their structure, composition, and interaction with other proteins, and post-translational modification affect the normal physiological processes of a cell [Citation10,Citation11]. Understanding the physiological functions of all the proteins and polypeptides is required to delineate their involvement in biological and molecular pathways in a particular type of cell. Proteomic-based studies on spermatozoa will explain the functional role of fertility-related proteins and the factors affecting their normal expression. Proteomic studies in earlier days were confined to checking the expression of proteins in different pathological conditions, but in the current proteomics era, sperm proteomics has been explored by various researchers to understand cellular pathways affecting male infertility [Citation11].
Advanced proteomic methodology and tools
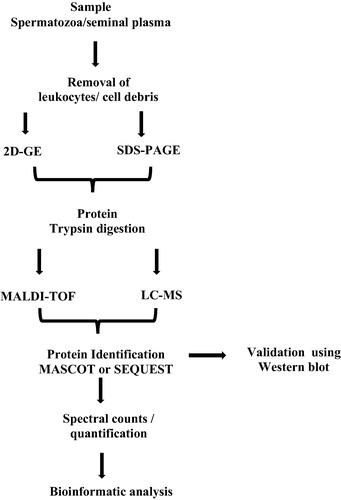
Previously, identification and quantification of multiple proteins at a single time were a challenging task. Development and introduction of new advanced tools and techniques have revolutionised the field of proteomics. In general, the protein techniques are classified into separation and identification techniques. Conventional separation techniques include separation of proteins in a given sample by sodium dodecyl sulphate (SDS)–PAGE based on their molecular weight. Similarly, two dimensional (2D)-gel electrophoresis is widely used for quantitative and qualitative proteins in a much more efficient manner based on the isoelectric focusing point and molecular weight of proteins. However, this technique is unable to generate a complete profile of all the proteins and is not sensitive enough to detect low-abundance proteins.
Current proteomic approaches are aimed at protein profiling, differential expression of proteins, localisation, and identification of post-translational modification, analysis of protein–protein interactions, and protein involvement in regulating biological and molecular pathways. Mass spectrometry (MS) techniques are able to overcome the omission in protein and peptide identification by measuring the m/z ratio. Initially the complexity of the proteins is broken down by 2D-gel electrophoresis and further MS characterises the proteins. It is an excellent technique to monitor and analyse thousands of peptides and proteins in a short time () [Citation12]. Current methodological advances in sperm proteomics using matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) and liquid chromatography (LC)–MS/MS techniques have led to increased throughput study of the sperm proteome [Citation13].
Quantitative proteomics
The relative quantity of the proteins is determined by comparing the number of spectra, termed spectral counts, used to identify each protein. The total numbers of mass spectra that match peptides to a particular protein (spectral counts or SpCs) are used to measure the abundance of proteins in the complex mixture. Normalisation of SpCs using the normalised spectral abundance factor (NSAF) approach is applied prior to relative protein quantification. Differentially expressed proteins (DEPs) are obtained by applying different constraints for significance tests and/or fold change thresholds based on the average SpC of the protein from multiple runs, as accurate quantification and determination of real biological change are a function of the absolute number of SpCs. The abundance of proteins can be classified as ‘High’, ‘Medium’, ‘Low’, or ‘Very Low’ based on their average SpCs. Different constraints for significance tests (P value) and/or fold change thresholds (or NSAF ratio) are applied for better statistical analysis [Citation14].
Bioinformatics analysis of proteomics data
Bioinformatics is a fusion of biological science with software-based computer science. It is mainly involved in the analysis of molecular scientific data using advanced software tools. Raw data obtained from the MALDI-TOF and LC–MS/MS techniques are subjected to bioinformatics analysis to distil the huge amount of data generated during proteomic studies into a more presentable form of results [Citation15]. Each protein analysis software works on specific algorithms and further analysis generates a list of proteins. Initially, MS/MS collision-induced dissociation (CID) spectra are analysed using data searching tools, such as Mascot, SEQUEST and X!Tandem to determine the SpCs. The proteins and peptides are then further matched with protein sequences, expressed sequence tags (EST) and DNA sequences that are available on global databases.
Gene names corresponding to the identified proteins are provided for functional annotations, whilst gene prediction programs are used to identify the proteins unavailable in the database based on their functional group [Citation16]. The next step is gene ontology (GO) analysis for the identified list of proteins using databases such as GO Term Finder and GO Term Mapper, which provide information for the proteins based on their function, localisation, structure, and biological function in cellular pathways. The genes/proteins are basically classified into cellular components, biological processes, and molecular functions. Interaction network and pathway analysis for the group of proteins is performed using proprietary software packages such as Ingenuity Pathway Analysis (IPA) and Metacore™ to identify the pathways, interactions, and cellular distribution of the proteins. Another online tool, Search Tool for the Retrieval of Interacting Genes/Proteins (STRING), displays the functional link between the protein–protein interactions amongst the proteins [Citation17].
Semen proteome
Semen is the most accepted and suitable sample for proteomic studies, as it contains thousands of proteins. Semen is composed of spermatozoa and protein-rich seminal fluid. Characterisation of proteins in spermatozoa and seminal plasma provides the function of fertility-related specific proteins [Citation18]. The male gamete with specialised functions is produced by a complex process known as spermatogenesis. Protein–protein interaction regulates the developmental process of spermatozoa [Citation19]. Using 1D-SDS–PAGE combined with the in-gel digestion coupled with MS analysis (GeLC–MS/MS) technique, Johnston et al. [Citation7] mapped 1760 proteins in human spermatozoa and also reported the abundance of 27 proteins present in the 26S proteasome complex of spermatozoa. Characterisation by the 2D-gel MALDI-TOF proteomic approach elucidated that proteins in the spermatozoa are involved in vital functions such as: energy production, apoptosis and oxidative stress (OS), cytoskeleton, flagella and cell movement, protein transport, and folding [Citation20]. A review by Amaral et al. [Citation21] using 30 different studies on sperm proteomics identified a total of 6198 proteins that constitute the sperm proteome, including protein characterisation studies from sperm head, tail, and membrane separately. Of these proteins, 898 were present in the head, 984 in the tail, and 532 in both locations [Citation22]. Functional analysis predicted sperm proteins were found to regulate important pathways such as: energy metabolism, protein metabolism, post-translational modifications, membrane trafficking, OS, DNA damage, and apoptosis [Citation21,Citation23].
Seminal plasma is heterogeneous as it is composed of secretions from the testis and accessory glands (including the prostate, seminal vesicles, epididymis, and Cowper’s gland), which provide a favourable environment for the spermatozoa [Citation24]. It has a rich protein concentration (35–55 g/L) and most seminal plasma proteins originate from accessory sex glands. Seminal plasma proteins are responsible for the coagulation–liquefaction process, making it complex for proteomic studies. Pilch and Mann [Citation25] reported the expression of 923 proteins in the seminal plasma and 70% of these proteins were present in spermatozoa [Citation23]. Jodar et al. [Citation22] identified 284 proteins including: TGF β1 (TGFB1), TGF β3 (TGFB3), antimicrobial peptide 1 (AMP1), serpin family A member 7 (SERPINA7), low-density lipoprotein receptor (LDLR), dystroglycan 1 (DAG1), disintegrin and metalloproteinase domain 10 (ADAM10), vitronectin (VTN), platelet-derived growth factor subunit A (PDGFA) and IGF-binding protein 2 (IGFBP2), which were specific only to seminal plasma. The same research group reviewed nine studies and reported 2064 proteins in the seminal plasma. Semenogelins (SEMG1 and SEMG2) were the two most abundant proteins (80%), whereas 10% of the proteins were from seminal extracellular vesicles, including epididymosomes and prostasomes [Citation22].
The testicular proteome too reflects the function of sperm, as spermatogenesis takes place in the testis. Guo et al. [Citation26] reported that the human testis expressed >1400 proteins and that 39 testis-specific proteins are involved in testicular function. Integrative analysis of the testis, sperm, and seminal plasma proteome reflected that 3901 sperm proteins are also present in the testes and 1213 proteins were associated with the acrosome and proteasome complex. Proteins belonging to molecular processes such as cell motility and peptidase activity that are essential for sperm motility and capacitation are transported from the seminal plasma to spermatozoa [Citation22].
Protein profiling and male infertility
In 2004, Pixton et al. [Citation68] reported the expression of at least 20 proteins that were altered in an infertile patient compared to fertile donors. Later, several proteomic studies were conducted to decipher the role of differentially expressed proteins in different infertility conditions related to sperm defects and abnormalities, i.e. varicocele, OS, mitochondrial dysfunction, sperm DNA damage, and testicular cancer. Ultimately, a set of potential protein biomarkers was proposed to distinguish infertility conditions as a cause of subcellular changes.
Semen abnormalities and proteomics
Azoospermia is associated with the absence of spermatozoa in the semen ejaculate and is the most discussed male infertility condition [Citation27]. Obstructive azoospermia (OA) is caused by physical obstruction or blockage in the male reproductive tract, whereas non-obstructive azoospermia (NOA) is mainly due to the arrest of the spermatogenesis process. Proteomic analysis of seminal plasma has shown the absence of certain proteins responsible for sperm function. Prostatic acid phosphatase/prostatic-specific acid phosphatase (ACPP) and kallikrein 3 (KLK3) proteins were absent in azoospermic patients [Citation28], and other proteins such as clusterin (CLU), zinc α2-glycoprotein 1 (AZGP1) and progestogen-associated endometrial protein/glycodelin S (PAEP) were also reported to be absent [Citation29]. Different proteomic studies were conducted to determine the differential expression of proteins in azoospermia [Citation24,Citation29–Citation31]. Testis-expressed protein 101 (TEX101) is characterised as the biomarker for azoospermia and extracellular matrix protein 1 (ECM1) was able to differentiate NOA and post-vasectomy men with a threshold value of 2.3 μg/mL [Citation32].
Asthenozoospermia patients are characterised by reduced progressive motility of spermatozoa (<32%). Proteins related to motility and energy metabolism are altered especially in spermatozoa. A similar proteome profile was exhibited by both asthenozoospermia and immature sperm [Citation33], and the DEPs were associated with energetic metabolism, protein folding/degradation, vesicle trafficking, and cytoskeleton. Dysregulation of protein tyrosine phosphatase, non-receptor type 14 (PTPN14), a tyrosine phosphatase protein involved in the regulation of sperm motility is seen in asthenozoospermic patients. Recently, Hashemitabar et al. [Citation34] identified 14 DEPs as potential protein biomarkers that are associated with vital sperm function. A similar study by Martinez-Heredia et al. [Citation35] also identified 17 DEPs. Alteration in the expression of the phosphorylated proteins such as heat shock proteins (HSPs) due to post-translational modification also had a negative impact on sperm motility [Citation36].
Globozoospermia, known as round-headed sperm syndrome, is associated with sperm abnormalities. Key proteins involved in spermatogenesis, sperm motility, and cytoskeleton organisation are differentially expressed in globozoospermia [Citation37]. Proteins related to acrosome formation were also altered, especially the proteins present in the perinuclear theca region [Citation38].
Another important and common sperm abnormality, oligoasthenozoospermia (OAT), is a male infertility factor with mitochondrial and chromosomal abnormalities. Proteomic profiling of patients with OAT identified a total of 2489 proteins from seminal plasma and their participation in the glycerolipid metabolism pathway [Citation39]. A study by Sharma et al. [Citation40,Citation41] reported the down-regulation of cystatin 3 (CST3) and up-regulation of KLK3 and SEMG1 sperm proteins in OAT semen samples. Another similar study also determined the down-regulation of epididymal secretory protein E1 (NPC2), galectin-3 binding protein (LGALS3BP), lipocalin 1 (LCN1), and prolactin induced protein (PIP) in oligoasthenozoospermia compared with normozoospermia [Citation42] ( [Citation29–Citation31,Citation34,Citation35,Citation39,Citation41,Citation79].
Table 1 Identification of DEPs involved in semen abnormalities using high-throughput techniques.
Male reproductive disorders and dysfunction
Varicocele is the abnormal dilation of the pampiniform venous plexus surrounding the spermatic cord involving the back flow of blood from the abdomen into the testis [Citation43]. It affects 35% and 81% of the primary and secondary infertile men, respectively [Citation44]. Even though patients with varicocele have normal semen parameters, their fertility status is likely to be compromised. This is due to disturbance in the spermatozoa at a molecular level. Nitric oxide metabolism involved in the generation of ROS was activated in patients with varicocele [Citation45], whereas varicocelectomy increased the expression of HSP family A (HSP70) member 5 (HSPA5), superoxide dismutase 1 (SOD1) and ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit (ATP5D) in spermatozoa [Citation46]. Seminal plasma proteins [calcium binding protein (CAB45) and cysteine-rich secretory protein 3 (CRISP3)] also showed altered expression in patients with varicocele [Citation47].
Extensive research has been carried out at our laboratory to decipher the role of DEPs in patients with both unilateral and bilateral varicocele. All these proteins were involved in spermatogenesis, mitochondrial dysfunction, energy metabolism, and acrosome reaction [Citation48–Citation51]. Comparative proteomic analysis identified 58 DEPs in bilateral varicocele [Citation48] and 38 proteins were unique in patients with unilateral varicocele [Citation50]. Another study comparing patients with unilateral and bilateral varicocele predicted glutathione S-transferase mu 3 (GSTM3), sperm protein associated with the nucleus, X chromosome, family member B1 (SPANXB1), Parkinson disease protein 7 (PARK7), proteasome subunit α8 (PSMA8), dihydrolipoamide dehydrogenase (DLD), SEMG1, and SEMG2 as potential biomarkers to differentiate unilateral from bilateral varicocele [Citation48]. Network and pathway analysis was able to show that 87% of the DEPs involved in major energy metabolism were associated with mitochondrial dysfunction [Citation48]. A list of potential biomarkers in different varicocele conditions is provided in [Citation13,Citation41,Citation48,Citation49,Citation66,Citation80].
Table 2 Identification of DEPs involved in male reproductive disorders and dysfunction using high-throughput techniques.
OS due to increased production of ROS in semen is the most cited cause of infertility [Citation52–Citation54]. Excessive production of ROS including the hydroxyl radical, peroxyl radicals, nitrous oxide, and nitroxyl anions results in prolonged OS, which can result in DNA damage [Citation55–Citation60]. High levels of oxidants also negatively impact sperm motility, count, morphology, and viability [Citation4,Citation61]. OS affects the stress responses and regulatory pathways in seminal plasma, as well as metabolic and stress responses in spermatozoa [Citation17]. Sharma et al. [Citation40] conducted proteomic studies on ROS-positive and -negative patients and proposed several potential biomarkers in both spermatozoa and seminal plasma (). Important proteins such as fibronectin 1 (FN1), macrophage migration inhibitory factor (MIF) and LGALS3BP were absent in the ROS-positive group but present in the ROS-negative group. However, in the seminal plasma, membrane metalloendopeptidase (MME) protein was absent in the ROS-negative group. A study by Intasqui et al. [Citation62] suggested mucin 5B, oligomeric mucus/gel-forming (MUC5B) as a potential biomarker in patients with OS with high lipid peroxidation.
Nuclear DNA damage to the spermatozoa affects the fertilisation process due to alteration in the sperm proteome. Intasqui et al. [Citation63] reported 23 and 71 overexpressed proteins in spermatozoa with high and low DNA damage, respectively. Overexpression of proteins had an impact on the triacylglycerol metabolism, energy production, protein folding, response to unfolded proteins, and cellular detoxification process. Certain proteins such as solute carrier family 2 member 14 (SLC2A14), phosphoglycerate kinase 2 (PGK2), outer dense fibre of sperm tails 1 (ODF1), CLU, voltage-dependent anion channel 2 (VDAC2), VDAC3, zona pellucida binding protein 2 (ZPBP2), and gastricsin (PGC) were identified as potential biomarkers. However, only nine and 21 proteins in the seminal plasma were differentially expressed in samples with low and high sperm DNA fragmentation, respectively [Citation64]. Cysteine-rich secretory protein LCCL domain-containing 1 (CRISPLD1), CRISPLD2, and retinoic acid receptor responder protein 1 (RARRES1) were identified as seminal plasma biomarkers and proteasome subunit α type A signalling was hyper-regulated affecting sperm motility, acrosome reaction, and capacitation [Citation65,Citation66].
Mitochondrial dysfunction is a result of the disturbance in the electron transport chain and energy metabolism process of the spermatozoa. Mitochondrial proteins play a major role in maintaining energy homeostasis of spermatozoa and its activity level determines normal sperm function. Annexin A7 (ANXA7) is a proposed biomarker for sperm mitochondrial dysfunction disorder [Citation66]. Mitochondrial dysfunction of sperm is more prominent in varicocele and it is implicated by alteration in the proteins involved in mitochondrial electron transport complex, energy metabolism, and sperm motility [Citation48].
Miscellaneous causes include those related to androgen deficiency, in which AZGP1, ACPP and PIP proteins involved in the catalytic and binding activities were found to be absent in androgen-deficient patients [Citation67]. A study by Pixton et al. [Citation68] identified that PIP and ODF2 proteins were overexpressed in spermatozoa that were unable to fertilise an oocyte. Whereas an inflammatory condition of epididymis known as epididymitis affected the expression of 35 sperm proteins [Citation69].
A recent in vitro study conducted on spermatozoa to check the effect of harmful xenoestrogen bisphenol-A revealed a change in the sperm proteome. A set of 24 proteins were differentially expressed on exposure to bisphenol-A and were involved in the activation of several kinase pathways in spermatozoa [Citation70].
Male reproductive cancer
Fertility preservation has become an essential process in patients being treated for cancer. Generally, sperm concentration is low in ∼50% of patients with testicular cancer and 40% of patients with Hodgkin’s disease. Infertility is one of the noted side-effects of cancer treatment. Cancer treatment causes severe damage to the gonads and DNA of germ cells, thus affecting the fertilisation process. Cancers related to the reproductive system not only decrease the immunity of the system, but also have harmful effects on spermatogenesis [Citation71]. Analysis of human testicular tissue using 2D-high-performance liquid chromatography–MS/MS detected that out of 7346 proteins, transmembrane protease, serine 12 (TMPRSS12); tubulin polymerisation promoting protein family member 2 (TPPP2); protease, serine 55 (PRSS55); doublesex and mab-3 related transcription factor 1 (DMRT1); piwi-like RNA-mediated gene silencing 1 (PIWIL1), and hemogen (HEMGN) were associated with cancer [Citation72]. Our laboratory was the first to identify 398 DEPs [including the overexpression of PSA, prostatic acid phosphatase (PAcP), zinc α2-glycoprotein (ZAG), and SEMG1 and SEMG2, as well as under expression of A-kinase anchoring protein 4 (AKAP4) and dynein axonemal heavy chain 17 (DNAH17)] in patients with testicular cancer using a global proteomic approach. Mitochondrial dysfunction, oxidative phosphorylation, and tricarboxylic acid cycle were the major pathways dysregulated in the spermatozoa of patients with testicular cancer [Citation73].
Biomarker identification and diagnostic approach
Biomarker screening and identification is a laborious process and the most important task that needs to be accomplished for the development of novel diagnostics or therapeutics. Biomarkers are biomolecules present in body fluids and alteration in their concentration or expression is indicative of a pathophysiological state. It is used as a tool for predicting prognosis, diagnosis, and treatment outcomes [Citation74]. In the past two decades, molecular biomarkers have gained importance in the field of medicine as diagnostic molecules due to their specificity, detectability, and availability for identification in the early stages of cellular or tissue damage [Citation75]. The translational research platform has improved much with the use of biomarkers in clinical diagnosis and treatment.
The main challenge faced in the field of male infertility is to understand the subcellular changes or causes that affect physiological function of spermatozoa due to its complex biological system. Proteomics has proven to be a promising tool for discovering biomarkers related to male infertility. Analysis of proteomic data using bioinformatics tools provides functional information about the proteins regulating biological pathways related to reproductive function [Citation76]. Clinical sperm proteomics serves as a useful platform and scientists have discovered various potential molecular biomarkers associated with various disorders of male infertility.
Future of proteomics in male infertility
The future of proteomics in male infertility depends on overcoming the limitation in analysing semen samples. High-throughput sperm proteomic studies have certain limitations due to the complexity of the sample. It is well explained by the presence of highly abundant proteins such as SEMGs, particularly in the seminal plasma [Citation45,Citation64]. These highly abundant peptides mask the detection of other proteins analysed by MS. Therefore, a rapid increase in sensitivity and resolution of analytical techniques is required [Citation77,Citation78]. Integration of the other ‘omics’ (transcriptomics and metabolomics) with proteomics should interlink the pathways affected in spermatozoa and provide unbiased results. Apart from the discoveries in basic research, its purpose is fulfilled by translating these findings into a clinical setting.
Conclusion
Proteomic studies on the highly differentiated spermatozoa have evaluated the proteins involved in infertility conditions. Most of the DEPs in infertile patients alter normal sperm function at a molecular level. Validation and screening of the identified potential protein biomarker using Western blot and ELISA will further strengthen the biomarker confirmation for a specific infertility condition. Furthermore, it will benefit infertile couples with personalised treatment by biomarker screening in patients. Finally, the use of all omic-based platforms in diagnosing male infertility will have a maximum clinical impact.
Conflict of interest
None.
Source of funding
None.
Notes
Peer review under responsibility of Arab Association of Urology.
1 Abbreviations: ACPP, prostatic acid phosphatase/prostatic-specific acid phosphatase; ANXA7, annexin A7; AZGP1, zinc α2-glycoprotein 1; CLU, clusterin; CRISP(1)(3),cysteine-rich secretory protein (1) (3); CRISPLD(1)(2), CRISP LCCL domain-containing (1) (2); CST3, cystatin 3; 2D, two dimensional; DEP, differentially expressed protein; FN1, fibronectin 1; GO, gene ontology; HIST1H2BA, histone cluster 1 H2B family member A; HSP, heat shock protein; HSPA(2)(5), HSP family A (HSP70) member (2) (5); KLK3, kallikrein 3; LC–(MS), liquid chromatography–(mass spectrometry); LGALS3BP, galectin-3 binding protein; MALDI-TOF, matrix-assisted laser desorption ionisation time-of-flight; MIF, macrophage migration inhibitory factor; NSAF, normalised spectral abundance factor; (N)OA, (non-) obstructive azoospermia; OAT, oligoasthenozoospermia; ODF(1), outer dense fibre of sperm tails (1) (2); OS, oxidative stress; PAEP, progestogen-associated endometrial protein/glycodelin S; PGK2, phosphoglycerate kinase 2; PIP, prolactin induced protein; ROS, reactive oxygen species; SDS, sodium dodecyl sulphate; SEMG(1)(2), semenogelin (1) (2); SOD1, superoxide dismutase 1; SpC, spectral count; TEX101, testis-expressed protein 101; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling; VDAC(2)(3), voltage-dependent anion channel (2) (3); ZPBP2, zona pellucida binding protein 2.
References
- A.AgarwalL.SamantaR.P.BertollaD.DurairajanayagamP.IntasquiSpringer briefs in reproductive biology: proteomics in human reproduction: biomarkers for millennials2016SpringerNew York
- World Health OrganizationWHO laboratory manual for the examination and processing of human semen5th ed.2010WHO pressSwitzerland
- A.AgarwalS.GuptaR.SharmaReactive oxygen species (ROS) measurementA.AgarwalS.GuptaR.SharmaAndrological evaluation of male infertility: a laboratory guide2016Springer International PublishingNew York155163
- A.AgarwalR.SharmaS.RoychoudhuryS.Du PlessisE.SabaneghMiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasmaFertil Steril1062016566573
- A.AgarwalS.GuptaR.SharmaMeasurement of DNA fragmentation in spermatozoa by TUNEL assay using bench top flow cytometerA.AgarwalS.GuptaR.SharmaAndrological evaluation of male infertility: a laboratory guide2016Springer International PublishingNew York181203
- M.A.BakerB.NixonN.NaumovskiR.J.AitkenProteomic insights into the maturation and capacitation of mammalian spermatozoaSyst Biol Reprod Med582012211217
- D.S.JohnstonJ.WootersG.S.KopfY.QiuK.P.RobertsAnalysis of the human sperm proteomeAnn N Y Acad Sci10612005190202
- M.A.BakerR.J.AitkenProteomic insights into spermatozoa: critiques, comments and concernsExpert Rev Proteomics62009691705
- M.A.BakerR.WitherdinL.HetheringtonK.Cunningham-SmithR.J.AitkenIdentification of post-translational modifications that occur during sperm maturation using difference in two-dimensional gel electrophoresisProteomics5200510031012
- M.MannO.N.JensenProteomic analysis of post-translational modificationsNat Biotechnol212003255261
- S.S.du PlessisA.H.KashouD.J.BenjaminS.P.YadavA.AgarwalProteomics: a subcellular look at spermatozoaReprod Biol Endocrinol920113610.1186/1477-7827-9-36
- K.E.BurnumS.L.FrappierR.M.CaprioliMatrix-assisted laser desorption/ionization imaging mass spectrometry for the investigation of proteins and peptidesAnnu Rev Anal Chem12008689705
- R.OlivaS.De MateoJ.CastilloR.AzpiazuJ.OriolaJ.L.BallescàMethodological advances in sperm proteomicsHum Fertil (Camb)132010263267
- A.AyazA.AgarwalR.SharmaM.ArafaH.ElbardisiZ.CuiImpact of precise modulation of reactive oxygen species levels on spermatozoa proteins in infertile menClin Proteomics122015410.1186/1559-0275-12-4
- N.LanG.T.MontelioneM.GersteinOntologies for proteomics: towards a systematic definition of structure and function that scales to the genome levelCurr Opin Chem Biol720034454
- T.ZhouZ.M.ZhouX.J.GuoBioinformatics for spermatogenesis: annotation of male reproduction based on proteomicsAsian J Androl152013594602
- A.AgarwalD.DurairajanayagamJ.HalabiJ.PengM.Vazquez-LevinProteomics, oxidative stress and male infertilityReprod Biomed Online2920143258
- M.W.DuncanH.S.ThompsonProteomics of semen and its constituentsProteomics Clin Appl12007861875
- R.SharmaA.AgarwalSpermatogenesis: an overviewA.ZiniA.AgarwalSperm chromatin: biological and clinical applications in male infertility and assisted reproduction2011SpringerNew York1944
- J.Martínez-HerediaJ.M.EstanyolJ.L.BallescàR.OlivaProteomic identification of human sperm proteinsProteomics6200643564369
- A.AmaralJ.CastilloJ.Ramalho-SantosR.OlivaThe combined human sperm proteome: cellular pathways and implications for basic and clinical scienceHum Reprod Update2020134062
- M.JodarA.Soler-VenturaR.OlivaMolecular Biology of Reproduction and Development Research Group2. Semen proteomics and male infertilityJ Proteomics1622017125134
- M.JodarE.SendlerS.A.KrawetzThe protein and transcript profiles of human semenCell Tissue Res36320168596
- I.BatruchI.LeckerD.KagedanC.R.SmithB.J.MullenE.Groberet alProteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital systemJ Proteome Res102011941953
- B.PilchM.MannLarge-scale and high-confidence proteomic analysis of human seminal plasmaGenome Biol72006R4010.1186/gb-2006-7-5-r40
- X.GuoP.ZhangR.HuoZ.ZhouJ.ShaAnalysis of the human testis proteome by mass spectrometry and bioinformaticsProteomics Clin Appl2200816511657
- A.HamadaR.SharmaS.S.du PlessisB.WillardS.P.YadavE.Sabaneghet alTwo-dimensional differential in-gel electrophoresis–based proteomics of male gametes in relation to oxidative stressFertil Steril992013e2 1216-26.e2
- M.Starita-GeribaldiS.PoggioliM.ZucchiniJ.GarinD.ChevallierP.Fenichelet alMapping of seminal plasma proteins by two-dimensional gel electrophoresis in men with normal and impaired spermatogenesisMol Hum Reprod72001715722
- M.Starita-GeribaldiF.RouxJ.GarinD.ChevallierP.FénichelG.PointisDevelopment of narrow immobilized pH gradients covering one pH unit for human seminal plasma proteomic analysisProteomics3200316111619
- K.YamakawaK.YoshidaH.NishikawaT.KatoT.IwamotoComparative analysis of interindividual variations in the seminal plasma proteome of fertile men with identification of potential markers for azoospermia in infertile patientsJ Androl282007858865
- A.P.DrabovichK.JarviE.P.DiamandisVerification of male infertility biomarkers in seminal plasma by multiplex selected reaction monitoring assayMol Cell Proteomics10M110201100412710.1074/mcp.M110.004127
- A.P.DrabovichA.DimitromanolakisP.SaraonA.SoosaipillaiI.BatruchB.Mullenet alDifferential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasmaSci Transl Med52013212ra16010.1126/scitranslmed.3006260
- A.AmaralC.PaivaC.Attardo ParrinelloJ.M.EstanyolJ.L.BallescàJ.Ramalho-Santoset alIdentification of proteins involved in human sperm motility using high-throughput differential proteomicsJ Proteome Res13201456705684
- M.HashemitabarS.SabbaghM.OrazizadehA.GhadiriM.BahmanzadehA proteomic analysis on human sperm tail: comparison between normozoospermia and asthenozoospermiaJ Assist Reprod Genet322015853863
- J.Martínez-HerediaS.de MateoJ.M.Vidal-TaboadaJ.L.BallescàR.OlivaIdentification of proteomic differences in asthenozoospermic sperm samplesHum Reprod232008783791
- P.P.ParteP.RaoS.RedijV.LoboS.J.D'SouzaR.Gajbhiyeet alSperm phosphoproteome profiling by ultra-performance liquid chromatography followed by data independent analysis (LC–MS E) reveals altered proteomic signatures in asthenozoospermiaJ Proteomics75201258615871
- T.T.LiaoZ.XiangW.B.ZhuL.Q.FanProteome analysis of round-headed and normal spermatozoa by 2-D fluorescence difference gel electrophoresis and mass spectrometryAsian J Androl112009683693
- C.Alvarez SedóV.Y.RaweH.E.ChemesAcrosomal biogenesis in human globozoospermia: immunocytochemical, ultrastructural and proteomic studiesHum Reprod27201219121921
- R.HerwigC.KnollM.PlanyavskyA.PourbiabanyJ.GreilbergerK.L.BennettProteomic analysis of seminal plasma from infertile patients with oligoasthenoteratozoospermia due to oxidative stress and comparison with fertile volunteersFertil Steril1002013e2 355-66 e2
- R.SharmaA.AgarwalG.MohantyS.S.Du PlessisB.GopalanB.Willardet alProteomic analysis of seminal fluid from men exhibiting oxidative stressReprod Biol Endocrinol1120138510.1186/1477-7827-11-85
- R.SharmaA.AgarwalG.MohantyR.JesudasanB.GopalanB.Willardet alFunctional proteomic analysis of seminal plasma proteins in men with various semen parametersReprod Biol Endocrinol1120133810.1186/1477-7827-11-38
- E.GiacominiB.UraE.GioloS.LuppiM.MartinelliR.C.Garciaet alComparative analysis of the seminal plasma proteomes of oligoasthenozoospermic and normozoospermic menReprod Biomed Online302015522531
- A.AgarwalA.HamadaS.C.EstevesInsight into oxidative stress in varicocele-associated male infertility: part 1Nat Rev Urol92012678690
- S.C.EstevesA.AgarwalAfterword to varicocele and male infertility: current concepts and future perspectivesAsian J Androl182016319322
- M.CamargoP.Intasqui LopesP.T.Del GiudiceV.M.CarvalhoK.H.CardozoC.Andreoniet alUnbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomyHum Reprod2820133346
- H.HosseinifarM.SabbaghianD.NasrabadiT.ModarresiA.V.DizajH.Gourabiet alStudy of the effect of varicocelectomy on sperm proteins expression in patients with varicocele and poor sperm quality by using two-dimensional gel electrophoresisJ Assist Reprod Genet312014725729
- P.Del GiudiceL.B.BelardinM.CamargoD.S.ZylbersztejnV.M.CarvalhoK.H.Cardozoet alDetermination of testicular function in adolescents with varicocoele–a proteomics approachAndrology42016447455
- A.AgarwalR.SharmaD.DurairajanayagamZ.CuiA.AyazS.Guptaet alSpermatozoa protein alterations in infertile men with bilateral varicoceleAsian J Androl1820164353
- A.AgarwalR.SharmaL.SamantaD.DurairajanayagamE.SabaneghProteomic signatures of infertile men with clinical varicocele and their validation studies reveal mitochondrial dysfunction leading to infertilityAsian J Androl182016282291
- A.AgarwalR.SharmaD.DurairajanayagamZ.CuiA.AyazS.Guptaet alDifferential proteomic profiling of spermatozoal proteins of infertile men with unilateral or bilateral varicoceleUrology852015580588
- A.AgarwalR.SharmaD.DurairajanayagamA.AyazZ.CuiB.Willardet alMajor protein alterations in spermatozoa from infertile men with unilateral varicoceleReprod Biol Endocrinol132015810.1186/s12958-015-0007-2
- R.J.AitkenJ.S.ClarksonT.B.HargreaveD.S.IrvineF.C.WuAnalysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermiaJ Androl101989214220
- R.K.SharmaA.AgarwalRole of reactive oxygen species in male infertilityUrology481996835850
- A.AgarwalR.K.SharmaK.P.NallellaA.J.ThomasJrJ.G.AlvarezS.C.SikkaReactive oxygen species as an independent marker of male factor infertilityFertil Steril862006878885
- A.AgarwalR.A.SalehM.A.BedaiwyRole of reactive oxygen species in the pathophysiology of human reproductionFertil Steril792003829843
- R.AitkenG.De IuliisOn the possible origins of DNA damage in human spermatozoaMol Hum Reprod162009313
- R.J.AitkenA.J.KoppersApoptosis and DNA damage in human spermatozoaAsian J Androl1320113642
- J.NovotnyN.AzizR.RybarJ.BrezinovaV.KopeckaR.Filipcikovaet alRelationship between reactive oxygen species production in human semen and sperm DNA damage assessed by Sperm Chromatin Structure AssayBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub1572013383386
- M.DorostghoalS.R.KazeminejadN.ShahbazianM.PourmehdiA.JabbariOxidative stress status and sperm DNA fragmentation in fertile and infertile menAndrologia492017e1276210.1111/and.12762
- X.WangR.K.SharmaS.C.SikkaA.J.ThomasJrT.FalconeA.AgarwalOxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertilityFertil Steril802003531535
- A.AgarwalS.M.WangClinical relevance of oxidation-reduction potential in the evaluation of male infertilityUrology10420178489
- P.IntasquiM.P.AntoniassiM.CamargoM.NichiV.M.CarvalhoK.H.Cardozoet alDifferences in the seminal plasma proteome are associated with oxidative stress levels in men with normal semen parametersFertil Steril1042015292301
- P.IntasquiM.CamargoP.T.Del GiudiceD.M.SpaineV.M.CarvalhoK.H.Cardozoet alUnraveling the sperm proteome and post-genomic pathways associated with sperm nuclear DNA fragmentationJ Assist Reprod Genet30201311871202
- P.IntasquiM.CamargoP.T.Del GiudiceD.M.SpaineV.M.CarvalhoK.H.Cardozoet alSperm nuclear DNA fragmentation rate is associated with differential protein expression and enriched functions in human seminal plasmaBJU Int1122013835843
- M.P.AntoniassiP.IntasquiM.CamargoD.S.ZylbersztejnV.M.CarvalhoK.H.Cardozoet alAnalysis of the functional aspects and seminal plasma proteomic profile of sperm from smokersBJU Int1182016814822
- P.IntasquiM.CamargoM.P.AntoniassiA.P.CedenhoV.M.CarvalhoK.H.Cardozoet alAssociation between the seminal plasma proteome and sperm functional traitsFertil Steril1052016617628
- D.MilardiG.GrandeF.VincenzoniA.GiampietroI.MessanaM.Castagnolaet alNovel biomarkers of androgen deficiency from seminal plasma profiling using high-resolution mass spectrometryJ Clin Endocrinol Metab99201428132820
- K.L.PixtonE.D.DeeksF.M.FleschF.L.MoseleyL.BjörndahlP.R.Ashtonet alSperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case reportHum Reprod19200414381447
- A.PilatzG.LochnitS.KarnatiA.Paradowska-DoganT.LangD.Schultheisset alAcute epididymitis induces alterations in sperm protein compositionFertil Steril1012014 1609–17.e1-5
- M.S.RahmanW.S.KwonJ.S.LeeS.J.YoonB.Y.RyuM.G.PangBisphenol-A affects male fertility via fertility-related proteins in spermatozoaSci Rep52015916910.1038/srep09169
- T.YokonishiT.OgawaCryopreservation of testis tissues and in vitro spermatogenesisReprod Med Biol1520162128
- M.LiuZ.HuL.QiJ.WangT.ZhouY.Guoet alScanning of novel cancer/testis proteins by human testis proteomic analysisProteomics13201312001210
- A.AgarwalE.TvrdaR.SharmaS.GuptaG.AhmadE.S.SabaneghSpermatozoa protein profiles in cryobanked semen samples from testicular cancer patients before treatmentFertil Steril104Suppl.201526010.1016/j.fertnstert.2015.07.817
- H.MischakG.AllmaierR.ApweilerT.AttwoodM.BaumannA.Benigniet alRecommendations for biomarker identification and qualification in clinical proteomicsSci Transl Med2201046ps4210.1126/scitranslmed.3001249
- J.R.KovacA.W.PastuszakD.J.LambThe use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertilityFertil Steril9920139981007
- Y.ZhuY.WuK.JinH.LuF.LiuY.Guoet alDifferential proteomic profiling in human spermatozoa that did or did not result in pregnancy via IVF and AIDProteomics Clin Appl72013850858
- K.WangC.HuangE.NiceRecent advances in proteomics: towards the human proteomeBiomed Chromatogr282014848857
- T.FuhrerN.ZamboniHigh-throughput discovery metabolomicsCurr Opin Biotechnol3120157378
- I.BatruchC.R.SmithB.J.MullenE.GroberK.C.LoE.P.Diamandiset alAnalysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertilityJ Proteome Res11201215031511
- R.SharmaA.AgarwalG.MohantyA.J.HamadaB.GopalanB.Willardet alProteomic analysis of human spermatozoa proteins with oxidative stressReprod Biol Endocrinol1120134810.1186/1477-7827-11-48