Abstract
Objective: To review sperm DNA fragmentation (SDF) testing as an important sperm function test in addition to conventional semen analysis. High SDF is negatively associated with semen quality, the fertilisation process, embryo quality, and pregnancy outcome. Over recent decades, different SDF assays have been developed and reviewed extensively to assess their applicability and accuracy as advanced sperm function tests. Amongst them, the standardisation of the terminal deoxynucleotidyl transferased UTP nick-end labelling (TUNEL) assay with a bench top flow cytometer in clinical practice deserves special mention with a threshold value of 16.8% to differentiate infertile men with DNA damage from fertile men.
Materials and methods: A systematic literature search was performed through the PubMed, Medline, and ScienceDirect databases using the keywords ‘sperm DNA fragmentation’ and ‘laboratory assessment’. Non-English articles were excluded and studies related to humans were only included.
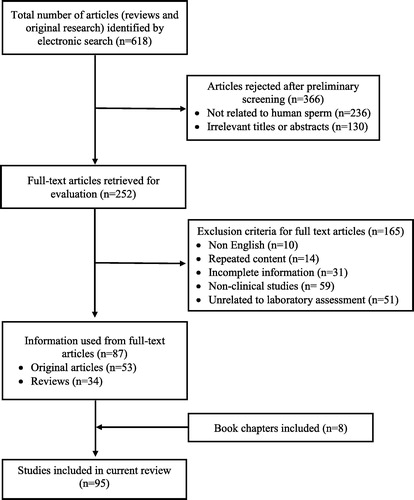
Results: Of the 618 identified, 87 studies (original research and reviews) and in addition eight book chapters meeting the selection criteria were included in this review. In all, 366 articles were rejected in the preliminary screening and a further 165 articles related to non-human subjects were excluded.
Conclusion: There are pros and cons to all the available SDF assays. TUNEL is a reliable technique with greater accuracy and as an additional diagnostic test in Andrology laboratories along with basic semen analysis can predict fertility outcome, and thus direct the choice of an assisted reproductive technology procedure for infertile couples. Also, the TUNEL assay can be used as a prognostic test and results are beneficial in deciding personalised treatment for infertile men.
Abbreviations:
- AO
- acridine orange
- ART
- assisted reproductive technology
- CMA3
- chromomysin A3
- dsDNA
- double-stranded DNA
- dUTP
- 2′-deoxyuridine 5′-triphosphate
- DFI
- DNA fragmentation index
- FITC
- Fluorescein isothiocyanate
- ICSI
- intracytoplasmic sperm injection
- IUI
- intrauterine insemination
- IVF
- in vitro fertilisation
- PI
- propidium iodide
- ROS
- reactive oxygen species
- SDF
- sperm DNA fragmentation
- ssDNA
- single-strand DNA
- TdT
- terminal deoxynucleotidyl transferase
- TUNEL
- terminal deoxynucleotidyl transferased UTP nick-end labelling
- SCD
- sperm chromatin dispersion
- SCSA
- sperm chromatin structure assay
Introduction
Infertility is prevalent in 9% of couples of reproductive age and is described as the inability to establish pregnancy within 12 consecutive months of unprotected intercourse. Amongst infertile couples, ∼20% is contributed by male factors alone [Citation1]. Continuous decline in male fertility over time, which cannot be attributed to any specific cause, results in idiopathic infertility [Citation2]. Various factors underlying male infertility include varicocele, oxidative stress, genetic abnormalities, systemic disease and infections, altered lifestyle, and exposure to xenobiotics [Citation3,Citation4]. All these factors can influence sperm DNA fragmentation (SDF), which acts as potential mediator for establishing an infertility status in men. Apart from these factors, the reproductive time line in men is one of the factors affecting semen parameters. Decline in the semen quality and increase in the SDF is observed after the ages of 35 and 40 years, respectively [Citation5Citation[6]–Citation7].
In current practice, male fertility status is evaluated indirectly based on the individual’s semen parameters. Conventional semen analysis is the first step in the assessment of infertile men and it reflects the overall functioning of all male reproductive organs [Citation8]. In general, semen volume, pH, sperm concentration, motility, vitality, and morphology are determined according to the WHO 2010 guidelines [Citation9]. Even though basic semen analysis is considered as the key investigation in all Andrology laboratories worldwide, it cannot accurately differentiate fertile from infertile men. Nearly 15% of infertile men have normal sperm parameters according to the WHO 2010 [Citation10]. This clearly indicates the presence of other subcellular and nuclear factors that have a major contribution towards male infertility that may not be identified by conventional semen analysis.
The nuclear component of the spermatozoa, especially sperm DNA integrity, is essential for normal fertilisation, implantation, pregnancy, and foetal development [Citation11,Citation12]. As a consequence of the high incidence of SDF in the men with idiopathic infertility [Citation13], recent research has focussed more on determining the clinical value of assessing SDF in male infertility and using SDF as an advanced sperm function test along with the conventional tests to evaluate the fertility status of the individual. The importance of the SDF assay has also been recognised in the latest AUA and European Association of Urology guidelines on male infertility [Citation14].
The present review evaluates the different laboratory techniques used for assessing SDF and the association between different SDF assays. The potential clinical use of the terminal deoxynucleotidyl transferased UTP nick-end labelling (TUNEL) assay to measure the SDF is discussed and the future use of SDF assays based on the assisted reproductive technology (ART) outcome are also reviewed.
Materials and methods
An extensive literature search of studies published until October 2017 was performed using the PubMed, Medline, and ScienceDirect databases. The search was limited to full articles in English and studies related to humans. The following primary keywords were used to extract the articles: ‘sperm DNA fragmentation’, ‘laboratory assessment’ and ‘male infertility’. Combination of the following keywords were also used to retrieve articles: ‘sperm DNA damage’, ‘TUNEL assay’, ‘oxidative stress’, ‘ART’, ‘laboratory test’, ‘male infertility’ and ‘advanced sperm test’. Search terms such as ‘SCSA test’, ‘SCD assay’ and ‘Comet assay’ were also used. Cross referencing was also referred to and used in the review process.
Results
A comprehensive literature review via electronic search of databases resulted in a total of 618 articles, comprising both review and original research articles. After preliminary screening, 252 articles were selected, which included different SDF studies from human sperm ( ). Subsequently, a further 165 were rejected from the 252 selected studies, of which 51 were not related to laboratory assessment of SDF. Finally, 87 full-text articles (original research and reviews) and eight book chapters met the inclusion criteria and were found to be eligible for the review.
SDF and damage
Chemical changes and structural changes in the germ cell DNA take place during the process of spermatogenesis. DNA is the most valuable genetic material and is highly condensed and compactly packed in spermatozoa in order to avoid damage. In general DNA is wrapped around the histone proteins and are replaced by highly basic protamines gradually for effective condensation of the sperm DNA [Citation15,Citation16], making the spermatozoa transcriptionally and translationally inactive [Citation17]. During this process torsional stress is incurred by double-stranded DNA (dsDNA). Therefore, nicks and breaks in the DNA are created and repair takes place for the proper rearrangement of chromatin [Citation18]. Failure to repair the nicks and its accumulative effect due to reduced protamination leads to DNA damage [Citation19].
Another cause of sperm DNA damage is reactive oxygen species (ROS) generated by immature spermatozoa. ROS attack the spermatozoa during epididymal transit causing damage to sperm DNA, either by activating the endonuclease or sperm caspases [Citation20]. Spermatozoa with poor chromatin packing or with high protamination are susceptible to ROS attack. In addition, SDF also occurs because of the poor disulphide cross-links in the mature spermatozoa due to alteration in the chromatin packaging. Epididymal sperms with lower levels of disulphide cross-linking are prone to DNA damage [Citation21,Citation22].
Both the intrinsic and extrinsic apoptosis pathways are activated in the spermatozoa on continuous exposure to high levels of ROS and reactive nitrogen species. Activation of pro-apoptotic factors by ROS result in leakage of cytochrome C from the mitochondrial membrane, which in turn activates intrinsic caspase cascade resulting in sperm DNA damage [Citation22Citation[23]–Citation24]. On the other hand, extrinsic apoptosis is initiated by the activation of Fas protein receptors present on the spermatozoa [Citation25]. These receptors are expressed in 10% of normozoospermic and 50% of oligozoospermic men [Citation26]. Leucocytes expressing ligands FasL bind to Fas receptors resulting in activation of pro-apoptotic proteins, which in turn disturb the mitochondrial pathways resulting in DNA damage. SDF is maximal when both the intrinsic and extrinsic apoptotic pathways are activated [Citation24,Citation27].
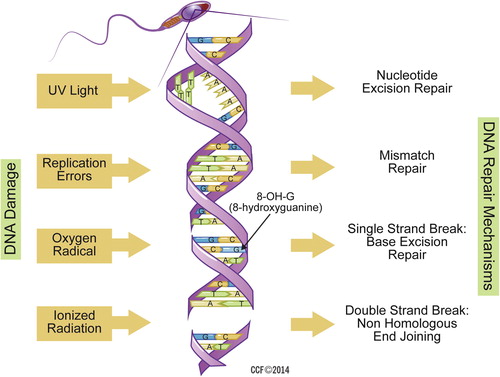
Other aetiological factors include: exposure to environmental toxins, caspase and endogenous endonucleases, replication error, ultraviolet rays, and ionised radiations. Both single-strand DNA (ssDNA) and dsDNA fragmentation are detrimental and make DNA unstable; however, dsDNA fragmentation is an irreversible damage and affects fertilisation and embryo development. To counteract the DNA damage process, DNA repair mechanisms such as nucleotide excision repair, mismatch repair, ssDNA and dsDNA break repair, helps in maintaining DNA integrity ( ). Defects in DNA repair mechanisms leads to abnormal sperm with a high degree of DNA damage [Citation24].
Different techniques of SDF assays for measuring DNA damage
A variety of assays have been developed to assess SDF. These tests either directly or indirectly measure sperm DNA integrity. All the tests are different from one another, thus their results are not inter-changeable, and the most commonly clinically used SDF tests are: sperm chromatin structure assay (SCSA), TUNEL, sperm chromatin dispersion (SCD), and the Comet assays.
Specimen preparation for SDF assay
A semen sample needs to be fixed immediately. For microscopic examination, neat semen samples are spread as thin smears on a glass slide and air dried. Further, they are fixed and can be used for staining the spermatozoa in assays such as toluidine blue staining and chromomysin A3 (CMA3) staining. In the case of samples analysed by flow cytometry the steps involved are: fixing, washing, permeabilising, staining, and analysis by flow cytometer. The most important factor affecting SDF during sample preparation is the prolonged incubation of semen samples, which increases SDF significantly after 2 h (8.81%, P = 0.004) and 3 h (10.76%, P < 0.001) [Citation28].
Toluidine blue staining
This microscopy assay assesses the integrity of the chromatin DNA of the spermatozoa. It stains the damaged chromatin nuclear structure of the spermatozoa and the degree of damage is visualised by optical microscopy.
Firstly, a thin smear is prepared with the semen sample and air dried. The smear is fixed in 96% ethanol-acetone solution of equal ratio for 30 min at 4 °C. Slides are treated with 0.1 M HCl for 5 min at 4 °C and stained with 0.05% toluidine blue stain for 10 min. Heads of the spermatozoa with high chromatin DNA integrity are stained blue and damaged DNA are stained purple. It is a rapid and simple assay [Citation29,Citation30].
CMA3 staining
CMA3 staining determines the damage to the DNA by measuring its protamination state. CMA3 binds more to the sperm DNA deficient of protamines, which is an indicator of poor DNA packing and damage [Citation31].
A semen sample smear is made on a glass slide, air dried, and fixed in glacial acetic acid–methanol (1:3) solution for 20 min at 4 °C. The stain containing 0.25 mg/mL of CMA3 with 10 mmol/L of MgCl2 is used for staining the spermatozoa. Stained slides are incubated overnight at 4 °C and examined for the presence of DNA damage. Spermatozoa with low protamination stain light yellow, whereas a bright yellow stain indicates high DNA damage due to increased protamination [Citation32]. A value of >30% DNA damage for semen samples determined by CMA3 assay has a significant effect in lowering fertilisation rates in ICSI [Citation33].
Acridine orange (AO) assay
The AO assay works on the simple principle that as the sperm DNA is subjected to acid denaturation it binds to the AO stain. AO bound to intact DNA is visualised as green and damaged DNA as red. The metachromatic shift in the fluorescence is analysed either by microscope or flow cytometer.
For microscopic examination the air-dried semen sample smears are fixed in Carnoy’s fixative for 2 h and followed by staining with AO for 5 min. In the case of flow cytometry analysis, 1 × 106 spermatozoa are fixed in 70% ethanol for 30 min and permeabilised using 0.1% Triton X-100 for 30 s. Then, spermatozoa stained with AO excited at 488-nm wavelength and the green fluorescence from the dsDNA and red fluorescence from ssDNA is measured [Citation34,Citation35]. The threshold value for this assay varies from 20% to 50% to differentiate fertile from infertile men [Citation35Citation[36]–Citation37].
SCSA
SCSA is a 30-year-old technique and the most widely studied test for sperm DNA damage. It is described as an indirect assay and the DNA is denatured either by heat or acidic solution to expose the DNA breaks. This assay detects the breaks in the ssDNA. Initially the DNA is denatured either by heat or acid treatment followed by staining with AO. AO bound to dsDNA emits green fluorescence, but when bound to ssDNA it emits red fluorescence. Stained cells are further evaluated with flow cytometry. Green-staining sperm have intact DNA, whilst red-staining sperm have denatured DNA [Citation38]. A clinical threshold for the DNA fragmentation index (DFI) of 30% was established based on the amount of red-staining sperm (DNA damage). This assay can be performed in both fresh and frozen samples.
It is also considered a simple test, with high repeatability in intra- and inter-laboratory results. Correlation between two certified laboratories (R 2 = 0.98) was high [Citation39]. The assay is also more precise and has a coefficient of variation of ∼1–3% [Citation40]. Threshold levels of 20–30% for the DFI have been determined by the SCSA, which is in contrast with the TUNEL assay ranging between a 4% and 36% DFI [Citation39].
SCD test/Halo
Fernández et al. [Citation41,Citation42] developed the SCD assay to measure SDF. It is an indirect technique in which intact DNA when loaded in agarose and denatured with acidic solution produces halos/chromatin dispersion due to the relaxed DNA, which is visualised by fluorescence microscopy [Citation43]. Such occurrence is not seen in spermatozoa with fragmented DNA. Sperm with non-dispersed chromatin (i.e. small halos) have fragmented DNA. The amount of sperm with non-dispersed chromatin is directly proportional to the ssDNA damage.
This test can be performed on both neat and washed semen samples. Initially, the sperm concentration is adjusted to 5–10 × 106/mL. A 20 µL sample of diluted spermatozoa is mixed with 80 µL 1% low melting agarose at 37 °C. On the pre-coated agarose slides, 50 µL of the aforementioned suspension is spread and allowed to solidify for 4 min at 4 °C and then covered with a coverslip. The second step is the denaturation of the DNA, done by immersing the sperm embedded in agarose into acidic solution (0.08 M HCl) for 7 min in a dark chamber at 22 °C, followed by treatment with neutralising and lysing solutions for 15 min at room temperature to arrest the denaturation. Further, it is washed in Tris–borate-ethylenediamine tetra-acetic acid (EDTA) buffer for 2 min and rehydrated in ascending grades of ethanol (70%, 90% and 100%). Finally the spermatozoa are stained with nuclear stain DAPI (4′,6-diamidino-2-phenylindole) and observed under a fluorescence microscope [Citation30,Citation42,Citation44].
Comet assay
In the Comet assay, also known as single-cell gel electrophoresis, the spermatozoa embedded in the agarose gel are lysed with detergent and migration of the fragmented DNA is appreciated as a tail, whilst intact DNA remains in the head. This technique was first introduced by Ostling and Johanson [Citation45] in 1984. During electrophoresis, small-stranded DNA moves out of the head further than large DNA strands. The intensity of the fluorescent staining and length of the tail is directly proportional to different degrees of DNA fragmentation within individual spermatozoon. This assay can detect multiple types of DNA fragmentation only in fresh semen samples and requires only 5000 spermatozoa, hence it can be easily performed even with oligozoospermic samples [Citation46].
Spermatozoa are dispersed individually and suspended in low-melting agarose at 37 °C. This mixture is placed on a microscopic slide and covered with a glass coverslip. These slides are placed at 4 °C to undergo solidification process followed by lysis of spermatozoa with buffer containing Triton X-100 detergent and proteinase K. Electrophoresis of the micro-slides in neutral buffer for 20 min at 25 V separates out the fragmented DNA from intact DNA towards the anode pole [Citation47]. Whereas in the case of the alkaline Comet assay, slides are placed in denaturing solution containing 0.03 M NaOH and 1 M NaCl for 2 min 30 s at 4 °C and electrophoresis carried out for 4 min in 0.03 M NaOH buffer at 20 V [Citation48]. After the completion of electrophoresis the slides are stained with SYBR Green I to visualise fragmented DNA under a fluorescence microscope. The results are analysed based on the tail length either manually or using specialised commercially available software [Citation30].
TUNEL assay: established clinical technique to measure SDF
Amongst all the current assays, determination of SDF in infertile men by TUNEL assay has gained clinical importance, as it targets the DNA strand breaks in the sperm DNA. SDF can be determined either by microscope or flow cytometer and can be performed with neat, washed or cryopreserved samples. However, the flow cytometry based assay is the most accurate due to its high sensitivity compared with the microscopic assay [Citation30]. Our centre, the American Center for Reproductive Medicine, Cleveland Clinic, Cleveland has standardised the TUNEL assay using a bench top flow cytometer (Accuri C6 flow cytometer; BD Biosciences, MI, USA) with reference values [Citation49].
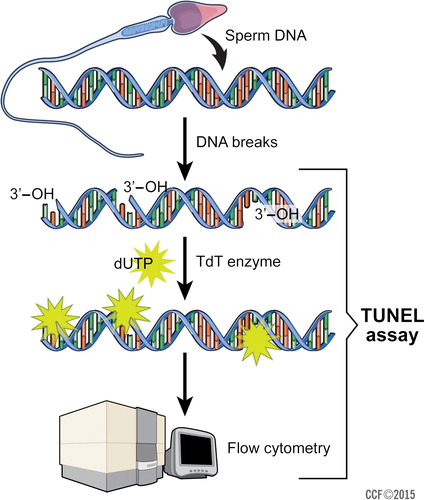
The TUNEL assay is based on the identification of DNA breaks by addition of template-independent DNA polymerase called terminal deoxynucleotidyl transferase (TdT) to the 3′hydroxyl (OH) breaks-ends of ssDNA and dsDNA. Fluorescein isothiocyanate (FITC) is conjugated with 2′-deoxyuridine 5′-triphosphates (dUTPs), the fluorescent signal measured by the flow cytometer is directly proportional to the DNA fragmentation in the analysed spermatozoa. The counter stain propidium iodide (PI), a red-fluorescent dye, is specifically used for nucleic acid staining ( ) [Citation50].
Procedure
Standardisation of the TUNEL assay for SDF has been reported recently with a bench top flow cytometer using the Apo-Direct kit (BD Pharmingen, CA, USA). A minimum of 2 × 106 sperms/mL are aliquoted from the liquefied semen sample and fixed in 1 mL paraformaldehyde (3.7%). Further, the spermatozoa are separated by centrifugation at 600g for 4 min and incubated in ice-cold 70% ethanol at −20 °C for 30 min. After incubation centrifuge again at 300g for 7 min to remove the supernatant without disturbing the sperm pellet. Add 1 mL of wash buffer to the pellet and vortex to re-suspend the sperm pellet. Centrifuge the tubes as in the previous steps to remove the wash buffer. Re-suspend the pellet in the 50 µL of staining solution. Along with the spermatozoa, FITC-dUTP staining is also done for the negative (6553LZ) and positive (6552LZ) control cells provided with the kit. The unreacted and leftover FITC-dUTP after 60 min incubation at 37 °C is removed from the solution by centrifugation (300g for 7 min), followed by rinsing with 1 mL rinse buffer. Finally, the cell pellet is re-suspended in 0.5 mL PI/RNase staining buffer and incubated at room temperature for 30 min.
SDF analysis is done on a BD Accuri C6 flow cytometer. The flow cytometer analyses cells based on their physical and fluorescence properties. Spermatozoa are passed through the flow channel and sorted based on the fluorescence signals generated by the stained cells. Each sample is run in duplicate along with the negative and positive controls. Laser detector FL1 (488 nm) with a standard 533/30 nm band pass detects green fluorescence FITC signals, whereas FL2 with a standard 675/25 nm band pass detects red fluorescence produced by PI. A minimum of 10 000 events are analysed and the spermatozoa positive for TUNEL are considered as DNA fragmented. Pre-installed user-friendly Zoom tool software is used to identify the percentage of SDF in the individual semen sample [Citation49].
Standardisation of reference value for TUNEL assay
Several studies have been carried out to establish a reference value for the TUNEL assay. A study by Sergerie et al. [Citation51], analysed samples from 66 infertile men and a reference value of 20%, with a specificity of 89.4% and sensitivity of 96.9% for the test was established, along with a high positive predictive value of 92.8%. Similarly, other studies have also reported threshold values of 12% [Citation49], 20% [Citation52] and 24.3% [Citation53] to differentiate infertile men with SDF from fertile men.
Inconsistency and high variability in the reference values for different types of TUNEL assays to assess SDF initiated research at the American Center for Reproductive Medicine to carry out intense studies to arrive at a threshold value to differentiate infertile men with DNA damage from controls. Initially, the test was carried out with 194 infertile men and the assay had much less inter- and intra-observer variability and inter-assay variability (<10%). A threshold value of 19.25% was established with a 64.9% sensitivity and 100% specificity for the assay to differentiate healthy donors from infertile men [Citation54]. As this study lacked clear established reference values and use of the instrument is difficult in clinical practice, a recent study with a large sample size of 261 infertile men defined a reference value of 16.8% with a high specificity of 91.6% and positive predictive value of 91.4% [Citation55] using a bench top Accuri C6 flow cytometer, making it more convenient for clinical laboratory use. A standardised protocol for the assessment of SDF using a bench top flow cytometer was made available for patients in the clinical laboratory [Citation49]. Further, our group has also attempted to standardise the TUNEL assay with another reference laboratory at Basel, Switzerland. The TUNEL assay had high correlation between the two centres (r = 0.94) and similarly the average SDF rates also had a strong positive correlation (r = 0.719) [Citation56]. Currently, experiments to standardise the TUNEL assay between two bench top units, the Accuri C6 flow cytometer and Accuri C6 Plus flow cytometer, has demonstrated the same threshold values for SDF (unpublished data). Overall, these standardisation studies allow researchers to compare results from different laboratories and also to establish reference ranges and improve the predictive value of the TUNEL assay.
Correlation amongst different SDF assays
Even though the comparison between direct (TUNEL) and indirect (SCSA) assays cannot be done, as TUNEL measures real DNA damage whereas SCSA detects the DNA damage after denaturation of DNA treated with acid solution [Citation57], certainly a correlation exists between the techniques when differentiating patients with high SDF from a control group based on the threshold values for each test separately. A meta-analysis by Cui et al. [Citation58] in 2015, reported that SDF assays had high diagnostic accuracy for identification of infertility with a sensitivity and specificity of 80% and 83% respectively having an area under the curve value of 0.92. The SCD and Comet assays had a combined sensitivity of 0.77 and 0.91, with a specificity of 0.91 and 0.84, but the TUNEL test had a pooled sensitivity and specificity of 0.77 and 0.91, respectively. Compared with the SCD and Comet assays, the TUNEL technique has greater accuracy in the detection and differentiation of men with SDF from a control group. Only a few such studies exist that analyse the correlation between the different types of SDF assays. Chohan et al. [Citation59] reported that the SCSA assay had a strong relationship with the TUNEL and SCD assays (r > 0.866; P < 0.001) in fertile and infertile patients for SDF. But no relationship with the AO test. Later, García‐Peiró et al. [Citation60], reported high correlations amongst TUNEL, SCSA and SCD assays in determining SDF in patients (n = 11) and control donors (n = 8). A comprehensive analysis by Ribas-Maynou et al. [Citation61], using different SDF assays identified a high correlation between SCD and SCSA, between SCD and TUNEL, and between SCSA and TUNEL, whilst, there was a moderate correlation between the alkaline Comet assay and SCD, between the alkaline Comet assay and SCSA, and between the alkaline Comet assay and TUNEL. However, there was no correlation between neutral Comet assay and the other assays. A study by Simon et al. [Citation62], identified a positive correlation between the Comet and TUNEL assays (r 2 = 0.126; P < 0.001) in couples undergoing ART. The TUNEL and SCSA assays exhibited similar results for SDF in infertile patients compared with a control population [Citation63]and there was a strong correlation for the TUNEL and SCSA assays [Citation64].
Inter-observer and laboratory variation for different SDF assays
Inter-observer and inter-laboratory variation in the available techniques has deterred the commercialisation of SDF assays. Each test has its own limitations and drawbacks. Even though the SCSA test has the least intra- and inter-laboratory variation, the test is yet to be commercialised [Citation65]. For other assays such as AO, CMA3 staining, toluidine blue staining, and SCD assay, inter-observer variability is the major impediment. In the case of the TUNEL assay, the variation has been minimised by standardisation of the assay protocol and the inter-laboratory variability was low for the TUNEL assay compared with the SCSA technique [Citation64].
Taking this to next level, 10 laboratories from the Florence consortium were involved in standardising the protocol for Comet, SCSA and TUNEL assays by analysing the same set of samples amongst all the laboratories. This was mainly aimed to determine the extent of correlation amongst the three tests and the degree of variation amongst the laboratories [Citation66].
Current status and future directions in SDF assay
In the modern era, about 2–4% of births in developed countries are the result of ART [Citation67] and sperm DNA testing has been highly recommended in clinical practice to select spermatozoa with high DNA integrity to achieve better fertilisation rates, as poor DNA integrity use in ART procedures is associated with decreased implantation and pregnancy rates [Citation68]. Although each SDF technique has its own limitations, prognostic values have been assigned for different assays [Citation69]. The TUNEL assay is considered to be the most simple, sensitive and reliable test for assessing SDF with low inter-observer variation [Citation70]. As we witness an increasing trend in fertility research, in the future the performance and accuracy of the SDF tests in defining the cause for male infertility may increase tremendously.
Male infertility factors such as advanced age, varicocele, idiopathic infertility, obesity, and testicular cancer have major influences on SDF rates ( ). In most ART procedures, sperm DNA damage determines the effect on the fertilisation rate and embryo quality. It also has a negative effect on the pregnancy rate by intrauterine insemination (IUI), IVF and ICSI resulting in low live-birth rate and increased miscarriages and spontaneous pregnancy loss (). Therefore, the use of SDF tests can assure an increase in the success rate in infertile couples undergoing ART procedures.
Table 1 Associated factors and effect of high SDF on ART outcomes.
Conclusion
Even though different tests are available to assess SDF, still they lack optimisation and clear-cut clinical reference values, which makes the routine use of the SDF assays controversial. Amongst the SDF assays, SCSA is considered as a simple indirect test but certain limitations still restrict its use and commercialisation. The TUNEL technique, being a direct assay, assesses SDF with greater accuracy and the standardisation and optimisation of the most commonly used TUNEL assay, with no intra-laboratory variation, will increase the positive predictive value and precise use of SDF testing in clinical scenarios to determine molecular factors underlying male infertility. As this is just the beginning of the use of SDF assays in clinical practice, in future more comprehensive studies may increase the scope of providing SDF testing to infertile couples for better management.
Conflict of interest
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- A.AgarwalL.SamantaR.P.BertollaD.DurairajanayagamP.IntasquiSpringer Briefs in Reproductive Biology: Proteomics in Human Reproduction: Biomarkers for Millennials2016SpringerNew York
- N.SwainG.MohantyL.SamantaIntasqui Paula. Proteomics and Male InfertilityA.AgarwalL.SamantaR.P.BertollaD.DurairajanayagamP.IntasquiSpringer Briefs in Reproductive Biology: Proteomics in Human Reproduction: Biomarkers for Millennials2016SpringerNew York2143
- E.TahmasbpourD.BalasubramanianA.AgarwalA multi-faceted approach to understanding male infertility: gene mutations, molecular defects and assisted reproductive techniques (ART)J Assist Reprod Genet31201411151137
- C.L.ChoS.C.EstevesA.AgarwalNovel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentationAsian J Androl182016186193
- D.B.DunsonD.D.BairdB.ColomboIncreased infertility with age in men and womenObstet Gynecol10320045156
- A.F.StewartE.D.KimFertility concerns for the aging maleUrology782011496499
- L.KimberlyA.CaseA.P.CheungS.SierraS.AlAsiriB.Carranza-MamaneAdvanced reproductive age and fertility: no. 269, November 2011Int J Gynaecol Obstet117201295102
- A.AgarwalS.GuptaR.SharmaBasic Semen AnalysisA.AgarwalS.GuptaR.SharmaAndrological Evaluation of Male Infertility A Laboratory Guide2016SpringerNew York3946
- World Health OrganizationWHO Laboratory Manual for the Examination and Processing of Human Semenfifth edn.2010WHO pressGeneva, Switzerland
- A.AgarwalS.S.AllamaneniSperm DNA damage assessment: a test whose time has comeFertil Steril842005850853
- M.BenchaibJ.LornageC.MazoyerH.LejeuneB.SalleGuerin J.FrançoisSperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcomeFertil Steril87200793100
- J.A.CollinsK.T.BarnhartP.N.SchlegelDo sperm DNA integrity tests predict pregnancy with in vitro fertilization?Fertil Steril892008823831
- K.OleszczukL.AugustinssonN.BayatA.GiwercmanBungum M Prevalence of high DNA fragmentation index in male partners of unexplained infertile couplesAndrology12013357360
- Jarow J, Sigman M, Kolettis P. Optimal evaluation of the Infertile Male: Best Practice Statement reviewed and validity confirmed 2011. Available at: http://www.auanet.org/guidelines/male-infertility-optimal-evaluation-(reviewed-and-validity-confirmed-2011), 2016. Accessed December 2017.
- D.PocciaRemodeling of nucleoproteins during gametogenesis, fertilization, and early developmentInt Rev Cytol1051986165
- G.Fuentes-MascorroH.SerranoA.RosadoSperm chromatinArch Androl452000215225
- R.R.HenkelD.R.FrankenSperm DNA fragmentation: Origin and impact on human reproductionJ Reprod Stem Cell Biotechnol201188108
- L.MarconG.BoissonneaultTransient DNA strand breaks during mouse and human spermiogenesis: new insights in stage specificity and link to chromatin remodelingBiol Reprod702004910918
- V.W.AokiL.LiuD.T.CarrellIdentification and evaluation of a novel sperm protamine abnormality in a population of infertile malesHum Reprod20200512981306
- D.SakkasJ.G.AlvarezSperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysisFertil Steril93201010271036
- E.GrecoF.ScarselliM.IacobelliL.RienziF.UbaldiS.FerreroEfficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoaHum Reprod202005226230
- E.K.SteeleN.McClureR.J.MaxwellS.E.LewisA comparison of DNA damage in testicular and proximal epididymal spermatozoa in obstructive azoospermiaMol Hum Reprod51999831835
- C.ShahaR.TripathiD.P.MishraMale germ cell apoptosis: regulation and biologyPhilos Trans R Soc Lond B Biol Sci365201015011515
- S.GunesM.Al-SadaanA.AgarwalSpermatogenesis, DNA damage and DNA repair mechanisms in male infertilityReprod Biomed Online312015309319
- A.AgarwalT.M.SaidRole of sperm chromatin abnormalities and DNA damage in male infertilityHum Reprod Update92003331345
- D.SakkasE.MariethozJ.C.St JohnAbnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathwayExp Cell Res2511999350355
- S.J.MartinC.P.ReutelingspergerA.J.McGahonJ.A.RaderR.C.van SchieD.M.LaFaceEarly redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and AblJ Exp Med182199515451556
- A.NabiM.A.KhaliliI.HalvaeiF.RoodbariProlonged incubation of processed human spermatozoa will increase DNA fragmentationAndrologia462014374379
- J.ErenpreisaJ.ErenpreissT.FreivaldsM.SlaidinaR.KrampeJ.ButikovaToluidine blue test for sperm DNA integrity and elaboration of image cytometry algorithmCytometry A5220031927
- A.AgarwalI.TsarevJ.ErenpreissT.M.SaidSperm chromatin assessmentD.K.GardnerA.WeissmanC.M.HowlesS.ZeevTextbook of Assisted Reproductive Techniques Fourth Edition: Volume 1: Laboratory Perspectives2012CRC PressBoca Raton, FL, USA
- G.C.ManicardiD.BizzaroD.SakkasBasic and Clinical Aspects of Sperm Chromomycin A3 AssayA.ZiniA.AgarawalSperm Chromatin Biological and Clinical Applications in Male Infertility and Assisted Reproduction2011SpringerNew York171179
- G.C.ManicardiP.G.BianchiS.PantanoP.AzzoniD.BizzaroU.BianchiPresence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibilityBiol Reprod521995864867
- D.SakkasF.UrnerD.BizzaroG.ManicardiP.G.BianchiY.ShoukirSperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo developmentHum Reprod13Suppl. 419981119
- K.HoshiH.KatayoseK.YanagidaY.KimuraA.SatoThe relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human spermFertil Steril661996634639
- A.ZiniM.A.FischerS.SharirB.ShayeganD.PhangK.JarviPrevalence of abnormal sperm DNA denaturation in fertile and infertile menUrology60200210691072
- D.P.EvensonL.K.JostD.MarshallM.J.ZinamanE.CleggK.PurvisUtility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinicHum Reprod14199910391049
- K.GopalkrishnanK.HurkadliV.PadwalD.BalaiahUse of acridine orange to evaluate chromatin integrity of human spermatozoa in different groups of infertile menAndrologia311999277282
- Z.DarżynkiewiczF.TraganosT.SharplessM.R.MelamedThermal denaturation of DNA in situ as studied by acridine orange staining and automated cytofluorometryExp Cell Res901975411428
- D.P.EvensonSperm Chromatin Structure Assay (SCSA®): 30 years of experience with the SCSA®A.ZiniA.AgarawalSperm Chromatin Biological and Clinical Applications in Male Infertility and Assisted Reproduction2011SpringerNew York125149
- D.P.EvensonSperm chromatin structure assay (SCSA®)Methods Mol Biol9272013147164
- J.L.FernándezL.MurielV.GoyanesE.SegrellesJ.GosálvezM.EncisoSimple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion testFertil Steril842005833842
- J.L.FernándezL.MurielM.T.RiveroV.GoyanesR.VazquezJ.G.AlvarezThe sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentationJ Androl2420035966
- M.K.AnkemE.MayerW.S.WardK.B.CummingsJ.G.BaroneNovel assay for determining DNA organization in human spermatozoa: implications for male factor infertilityUrology592002575578
- H.PratapS.Y.HottigoudarK.S.NichanahalliP.ChandAssessment of sperm deoxyribose nucleic acid fragmentation using sperm chromatin dispersion assayJ Pharmacol Pharmacother820174549
- O.OstlingK.J.JohansonMicroelectrophoretic study of radiation-induced DNA damages in individual mammalian cellsBiochem Biophys Res Commun1231984291298
- N.P.SinghD.B.DannerR.R.TiceM.T.McCoyG.D.CollinsE.L.SchneiderAbundant alkali-sensitive sites in DNA of human and mouse spermExp Cell Res1841989461470
- N.P.SinghM.T.McCoyR.R.TiceE.L.SchneiderA simple technique for quantitation of low levels of DNA damage in individual cellsExp Cell Res1751988184191
- J.Ribas-MaynouA.García-PeiróC.AbadM.J.AmengualJ.NavarroJ.BenetAlkaline and neutral Comet assay profiles of sperm DNA damage in clinical groupsHum Reprod272012652658
- A.AgarwalS.GuptaR.SharmaMeasurement of DNA Fragmentation in Spermatozoa by TUNEL Assay Using Bench Top Flow CytometerA.AgarwalS.GuptaR.SharmaAndrological Evaluation of Male Infertility A Laboratory Guide2016SpringerNew York181203
- R.SharmaJ.MasakiA.AgarwalSperm DNA fragmentation analysis using the TUNEL assayMethods Mol Biol9272013121136
- M.SergerieG.LaforestL.BujanF.BissonnetteG.BleauSperm DNA fragmentation: threshold value in male fertilityHum Reprod20200534463451
- M.BenchaibV.BraunJ.LornageS.HadjB.SalleH.LejeuneSperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive techniqueHum Reprod18200310231028
- R.HenkelE.KierspelM.HajimohammadT.StalfC.HoogendijkC.MehnertDNA fragmentation of spermatozoa and assisted reproduction technologyReprod Biomed Online72003477484
- R.K.SharmaE.SabaneghR.MahfouzS.GuptaA.ThiyagarajanA.AgarwalTUNEL as a test for sperm DNA damage in the evaluation of male infertilityUrology76201013801386
- R.SharmaG.AhmadS.C.EstevesA.AgarwalTerminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality controlJ Assist Reprod Genet332016291300
- S.RibeiroR.SharmaS.GuptaZ.CakarC.De GeyterA.AgarwalInter-and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentationAndrology52017477485
- R.HenkelC.F.HoogendijkP.J.BouicT.F.KrugerTUNEL assay and SCSA determine different aspects of sperm DNA damageAndrologia422010305313
- Z.L.CuiD.Z.ZhengY.H.LiuL.Y.ChenD.H.LinFeng-HuaLanDiagnostic accuracies of the TUNEL, SCD, and Comet based sperm dna fragmentation assays for male infertility: a meta-analysis studyClin Lab612015525535
- K.R.ChohanJ.T.GriffinM.LafromboiseC.J.De JongeD.T.CarrellComparison of chromatin assays for DNA fragmentation evaluation in human spermJ Androl2720065359
- A.García-PeiróM.Oliver-BonetJ.NavarroC.AbadM.GuitartM.J.AmengualDynamics of sperm DNA fragmentation in patients carrying structurally rearranged chromosomesInt J Androl342011e546e553
- J.Ribas-MaynouA.García-PeiróA.Fernández-EncinasC.AbadM.J.AmengualE.PradaComprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assayAndrology12013715722
- L.SimonL.LiuK.MurphyS.GeJ.HotalingK.I.AstonComparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatmentHum Reprod292014904917
- E.WilandM.FraczekM.OlszewskaM.KurpiszTopology of chromosome centromeres in human sperm nuclei with high levels of DNA damageSci Rep620163161410.1038/srep31614
- C.LeSaintL.VingataraminS.AlixS.PhillipsA.ZiniJ.I.KadochCorrelation between two sperm DNA fragmentation tests (TUNEL and SCSA) and evaluation of TUNEL assay inter-lab variabialityFertil Steril106Suppl.2016e297
- M.B.ShamsiS.N.ImamR.DadaSperm DNA integrity assays: diagnostic and prognostic challenges and implications in management of infertilityJ Assist Reprod Genet28201110731085
- A.ZiniO.AlbertB.RobaireAssessing sperm chromatin and DNA damage: clinical importance and development of standardsAndrology22014322325
- U.PaaschS.GrunewaldH.GlanderSperm selection in assisted reproductive techniquesSoc Reprod Fertil Suppl652007515525
- R.K.SharmaT.SaidA.AgarwalSperm DNA damage and its clinical relevance in assessing reproductive outcomeAsian J Androl62004139148
- J.GosálvezC.López-FernándezJ.L.FernándezS.C.EstevesS.D.JohnstonUnpacking the mysteries of sperm DNA fragmentation: ten frequently asked questionsJ Reprod Biotechnol Fertil4201510.1177/2058915815594454
- C.L.ChoA.AgarwalA.MajzoubS.C.EstevesFuture direction in sperm DNA fragmentation testingTransl Androl Urol6Suppl. 42017S525S526
- S.BellocM.BenkhalifaM.Cohen-BacrieA.DalleacE.AmarA.ZiniSperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motilityFertil Steril101201415881593
- M.DasN.Al-HathalM.San-GabrielS.PhillipsI.J.KadochF.BissonnetteHigh prevalence of isolated sperm DNA damage in infertile men with advanced paternal ageJ Assist Reprod Genet302013843848
- A.ZiniG.DohleAre varicoceles associated with increased deoxyribonucleic acid fragmentation?Fertil Steril96201112831287
- E.TvrdaA.AgarwalN.AlkuhaimiMale reproductive cancers and infertility: a mutual relationshipInt J Mol Sci16201572307260
- O.StåhlJ.EberhardK.JepsonM.SpanoM.CwikielE.Cavallin-StåhlThe impact of testicular carcinoma and its treatment on sperm DNA integrityCancer100200411371144
- C.Esquerré-LamareF.IsusN.MoinardL.BujanSperm DNA fragmentation after radioiodine treatment for differentiated thyroid cancerBasic Clin Androl252015810.1186/s12610-015-0024-1
- V.M.IommielloE.AlbaniA.Di RosaA.MarrasF.MenduniG.MorrealeEjaculate oxidative stress is related with sperm DNA fragmentation and round cellsInt J Endocrinol2015201532190110.1155/2015/321901
- A.AgarwalA.MulgundS.AlshahraniM.AssidiA.M.AbuzenadahR.SharmaReactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermiaReprod Biol Endocrinol12201412610.1186/1477-7827-12-126
- S.CablerA.AgarwalM.FlintS.S.du PlessisObesity: modern man's fertility nemesisAsian J Androl122010480489
- N.O.McPhersonM.LaneMale obesity and subfertility, is it really about increased adiposity?Asian J Androl172015450458
- J.D.MeekerS.EhrlichT.L.TothD.L.WrightA.M.CalafatA.T.TrisiniSemen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinicReprod Toxicol302010532539
- S.SepaniakT.ForgesH.GerardB.FoliguetM.C.BeneP.Monnier-BarbarinoThe influence of cigarette smoking on human sperm quality and DNA fragmentationToxicology22320065460
- R.SharmaK.R.BiedenharnJ.M.FedorA.AgarwalLifestyle factors and reproductive health: taking control of your fertilityReprod Biol Endocrinol1120136610.1186/1477-7827-11-66
- M.SpanòJ.P.BondeH.I.HjøllundH.A.KolstadE.CordelliG.LeterSperm chromatin damage impairs human fertilityFertil Steril7320004350
- L.MurielM.MeseguerJ.L.FernándezJ.AlvarezJ.RemohíA.PellicerValue of the sperm chromatin dispersion test in predicting pregnancy outcome in intrauterine insemination: a blind prospective studyHum Reprod212005738744
- E.H.DuranM.MorshediS.TaylorS.OehningerSperm DNA quality predicts intrauterine insemination outcome: a prospective cohort studyHum Reprod17200231223128
- V.S.RilchevaN.P.AyvazovaL.O.IlievaS.P.IvanovaE.I.KonovaSperm DNA integrity test and assisted reproductive technology (ART) outcomeJ Biomed Clin Res920162129
- M.CissenM.V.WelyI.ScholtenS.MansellJ.P.BruinB.W.MolMeasuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysisPLoS One112016e016512510.1371/journal.pone.0165125
- L.SimonG.BrunborgM.StevensonD.LuttonJ.McManusS.E.LewisClinical significance of sperm DNA damage in assisted reproduction outcomeHum Reprod25201015941608
- I.D.MorrisSperm DNA damage and cancer treatmentInt J Androl252002255261
- M.VirroD.EvensonSperm chromatin structure assay (SCSA®) related to blastocyst rate, pregnancy rate, and spontaneous abortion in IVF and ICSI cyclesFertil Steril79Suppl. 220031610.1016/S0015-0282(03)00109-2
- M.H.Nasr-EsfahaniM.SalehiS.RazaviM.AnjomshoaS.RozbahaniF.MoulaviEffect of sperm DNA damage and sperm protamine deficiency on fertilization and embryo development post-ICSIReprod Biomed Online112005198205
- L.SimonI.ProutskiM.StevensonD.JenningsJ.McManusD.LuttonSperm DNA damage has a negative association with live-birth rates after IVFReprod Biomed Online2620136878
- A.OsmanH.AlsomaitS.SeshadriT.El-ToukhyY.KhalafThe effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysisReprod Biomed Online302015120127
- L.RobinsonI.D.GallosS.J.ConnerM.RajkhowaD.MillerS.LewisThe effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysisHum Reprod27201229082917