Abstract
Objective: To evaluate the detailed vascular anatomy of the spermatic cord during subinguinal microscopic varicocelectomy and to assess the outcome of the cases with regard to varicocele recurrence and hydrocele formation.
Patients and methods: In all, 100 varicocele cases including 74 left-sided and 26 bilateral, comprising 126 spermatic cord units with clinically palpable varicoceles underwent microscopic subinguinal varicocelectomy. Detailed description of vascular anatomy of the spermatic cords was reported. The number of spermatic, cremasteric, and inguinal veins was recorded. A record of testicular arteries and lymphatics was noted. Testicular delivery was done in all the cases and assessment of the gubernacular veins was reported. The patients underwent clinical evaluation, as well as scrotal Doppler ultrasonography, to detect varicocele recurrence and hydrocele formation. The mean (range) postoperative evaluation period was 6 (3–12) months.
Results: The mean number of spermatic veins was 14 on both sides. The mean number of spermatic arteries on both sides was 1.3. For lymphatics, the mean number was around three on both sides. The gubernacular veins were noted in 75% of the cases on the left side (mean number of 1.2) and in 85% on the right-side, (mean number of 1). The mean number of cremasteric veins on the left and right sides was 1.4 and 1.2, respectively. Finally, inguinal floor vessels were noted in 9% on the left-side and were not seen in the right-side cases. The incidence of varicocele recurrence was 2% and for hydrocele that was not clinically significant was 0.07%.
Conclusion: Microscopic subinguinal varicocelectomy accurately evaluated the detailed vascular anatomy of the spermatic cord, achieving excellent surgical outcome with minimal varicocele recurrence and hydrocele formation. Microscopic subinguinal varicocelectomy should be the ‘gold standard’ for varicocelectomy.
Abbreviation:
Introduction
Varicocele is found in ∼15% of the general male population [Citation1]. The European Association of Urology recommend varicocele repair for adults with clinical varicocele with infertility or abnormal semen quality and for adolescents with progressive failure of testicular development [Citation2]. The best treatment method should include elimination of the varicocele and should carry a low risk of complications. Therefore, the ideal technique should involve ligation of all internal and external spermatic veins with preservation of spermatic arteries and lymphatic vessels. Surgical management of varicocele can be done by retroperitoneal, inguinal, subinguinal, or laparoscopic approaches or by percutaneous embolisation [Citation3,Citation4]. Conventional techniques for varicocele repair are associated with substantial risks of hydrocele formation, ligation of the testicular artery, and varicocele recurrence. Conventional varicocelectomy is complicated by a postoperative recurrence rate of 5–20% in patients with varicocele [Citation5Citation[6]–Citation7]. The use of microscopy has significantly improved the outcome of varicocelectomy [Citation8]. Knowledge of the vascular anatomy of the spermatic cord is helpful in improving the outcome of varicocelectomy [Citation9]. In the present study, we sought to describe the vascular anatomy of the spermatic cord during microscopic subinguinal varicocelectomy. The purpose of the present study was to increase the awareness amongst urologist performing varicocelectomy of the detailed anatomy that needs to be considered when performing such an operation (– ).
Patients and methods
Patients who presented to our clinic complaining of scrotal pain, infertility or scrotal swelling and suspected to have varicocele were clinically evaluated by a single physician (A.A). They were evaluated in a standing position for 3 min and presence of varicocele, with its grade, was recorded. Then patients were evaluated with colour Doppler ultrasonography (US) of the scrotum. The diagnosis of varicocele was made when the vein diameter was >2 mm with reflux, without or with Valsalva. Semen tests were done for all the patients but not included in this study, as it was intended to describe the anatomy and measure the outcome of varicocele recurrence or hydrocele.
Ethical considerations
All patients were consented; the study was approved by Institutional Review Board according to the Declaration of Helsinki as a statement of ethical principles to provide guidance to physicians and other participants in medical research involving human subjects.
Protocol design
The study was a prospective clinical study and the study period was over 5 years from 2010 to 2015. The inclusion criteria were clinically palpable varicocele with the veins’ diameter on Doppler US of >2 mm; exclusion criteria were subclinical varicocele. The patients were followed-up as follows: at 1 week, and 1, 3, 6 and 12 months postoperatively. At each follow-up visit a physical examination of the wound and scrotum was performed, as well as scrotal US with Doppler to assess for varicocele recurrence and hydrocele. The mean (range) follow-up was 6 (3–12) months. The indications for varicocelectomy were infertility, testicular pain or reduced testicular volume.
Surgical technique
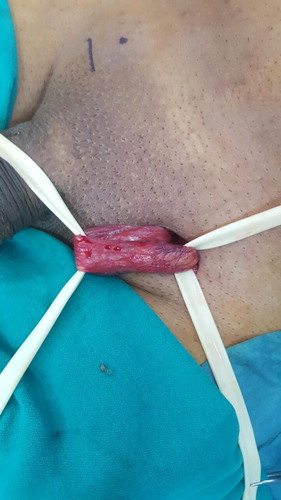
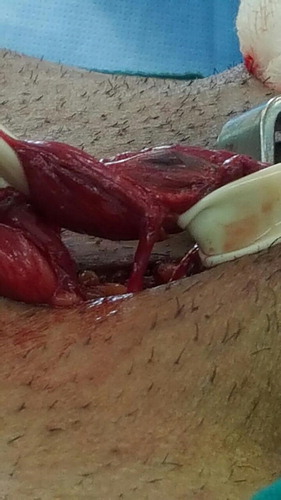
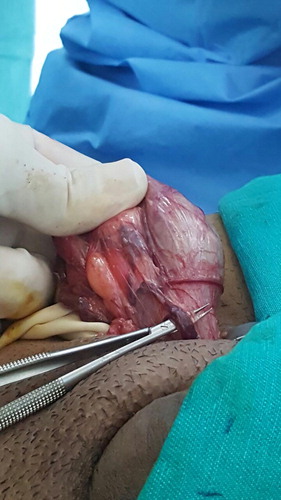
The operations were done under general anaesthesia and by single surgeon (A.A.). The surgical technique was according to Goldstein et al. [Citation10] and involved a 2.5–3 cm subinguinal incision. Then the cord was dissected and freed proximally and distally. Two Penrose drains were placed underneath for retraction. A microscope (Zeiss, Oberkochen, Germany) was used with ×7–15 magnification. A micro-Doppler probe 20 MHz (VTI-USA) was used to help identify the testicular, cremasteric, and even vassal arteries. The testicle was delivered in all cases either initially or after cord dissection. A detailed description of the vascular anatomy of the spermatic cords was reported, which included the number of spermatic, cremasteric, and inguinal veins. Also, the size of the spermatic veins was assessed and categorised as: ‘large’ (>2 mm) or ‘the rest’ (<2 mm). The number of testicular arteries and lymphatics were also noted. Testicular delivery was done in all the cases and assessment of gubernacular veins was reported.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS® version 22; SPSS Inc., IBM Corp., Armonk, NY, USA) was used for statistical analysis to compare the number of large spermatic veins (>2 mm) with the rest of the veins (<2 mm) and to compare the difference between the mean number of vessels between the right and left sides of the spermatic cord.
Results
In all, 100 patients with a median (range) age of 28 (17–50) years were included; there was one adolescent aged 17 years. The cases included 74 left-sided and 26 bilateral, comprising 126 spermatic cord units with bilateral clinically palpable varicoceles who underwent microscopic subinguinal varicocelectomy. The mean (range) number of external spermatic veins was 1.2 (1–4) on the left side and 1 (1–3) on the right side ( ). The mean (range) number of internal spermatic veins on the left side was 14 (3–38) with a mean (range) of 3 (1–7) large veins. Large veins included any vein with a diameter of >2 mm and all measurements were taken intraoperatively with a sterile ruler. The mean (range) number of internal spermatic veins on the right side was also 14 (11–25), and the mean (range) number of large veins on the right side was 2 (1–8). The mean number of spermatic arteries on both sides was 1.3. For the lymphatics, the mean number was ∼ 3 on both sides. Gubernacular veins were noted in 75% of the cases on the left side, with a mean number of 1.2, and in 85% on the right-side, with a mean of 1. The mean number of cremasteric veins on the left and right sides was 1.4 and 1.2, respectively. Finally, inguinal floor vessels were noted in 9% on the left side and were not seen in the right-side cases. The number of veins was not correlated with the degree of varicocele. There was no correlation between the number of veins in adults and adolescents. Varicocele recurrence was noted in three cases with an incidence of 2% and mild hydrocele, which was not clinically obvious but only detected by US, was found in one case with an incidence of 0.07%.
Table 1 Vessels detected during microscopic subinguinal varicocelectomy.
There was a significant difference between the mean number of large veins and the mean number of the rest of the veins (P < 0.05) in the same spermatic cord. Conversely, there was no significant difference between the mean number of vessels including arteries, veins and lymphatics between the right and left spermatic cords.
Discussion
A microsurgical approach to varicocelectomy was first described by Goldstein et al. [Citation10] in 1992. Microsurgical varicocelectomy is considered the ‘gold standard’ technique for treating varicocele in both adults and adolescents, due to relatively more favourable outcomes and lower postoperative recurrence and complication rates [Citation11,Citation12,Citation9,Citation13]. Optical magnification allows identification of all spermatic cord structures with inguinal and subinguinal approaches to varicocelectomy. The ability to identify these anatomical structures effectively is the basis for the recommendation by the AUA Best Practice Policy Committee that optical magnification be used during varicocele repair [Citation2]. In our present study, we used a meticulous microsurgical subinguinal dissection of the spermatic cord. In the subinguinal approach, the external oblique aponeurosis is not opened and the cord is isolated by dissection at the level just inferior to the external inguinal ring. So, the subinguinal approach is associated with less pain and a rapid recovery in comparison with the inguinal approach [Citation11,Citation12].
The main route of venous drainage of the testis is the internal spermatic vein and the external pudendal vein. The cremasteric and vasal veins are smaller collaterals [Citation9]. The testicular veins emerge posteriorly from the testis, drain the epididymis and unite to form the pampiniform plexus. Varicocele develops from a reversal of blood flow within the internal spermatic and cremasteric veins [Citation14].
In the inguinal canal the pampiniform plexus is drained by three or four veins, which run into the abdomen through the deep inguinal ring [Citation15]. According to Marmar et al. [Citation11], a total of 11.1 internal spermatic veins per dissection were identified subinguinally. A mean of 0.4 internal spermatic veins of ≥5 mm in diameter per cord was identified subinguinally. A corresponding increase in the mean number of internal spermatic veins of ≤2 mm in diameter was noted with 7.9 at the subinguinal level [Citation11]. In the present study, the mean number of spermatic veins was 14 on both sides. The microscope identifies small spermatic veins but, the increased number of veins in the subinguinal region can make surgical procedures more tedious and technically difficult than with an inguinal approach.
We also found that the mean number of cremasteric veins on the left and right sides was 1.4 and 1.2, respectively. Inguinal floor vessels were noted in 9% on the left side and were not seen in the right-side cases. Gubernacular veins were noted in 75% of the cases on the left side, with a mean number of 1.2, and in 85% on the right-side, with a mean of 1. Veins exiting through the gubernaculum were commonly seen in subinguinal studies on average in 71%. It is unclear exactly what role the gubernacular veins have in the pathogenesis of varicoceles and in postoperative varicocele recurrence. In a study by Murray et al. [Citation15], examining varicocele recurrence, scrotal collaterals were presumed to exist in 7% of recurrent varicoceles.
Recurrence after varicocele repair is the most variable complication, as it depends largely on the technique used and the use of magnification. Recurrence rate varies from 0% to 35% [Citation15]. Sayfan et al. [Citation16] demonstrated by phlebography that three of the four postoperative varicocele recurrences in their series were due to dilated external spermatic veins in the presence of total internal spermatic vein occlusion. Another study, involving venography, also demonstrated recurrences to be caused by peri-arterial, parallel inguinal, mid-peritoneal, gubernacular and trans-scrotal collateral veins [Citation17]. The most common cause of persistent or recurrent varicocele after surgical repair involves the internal spermatic veins; redundancies of the gonadal veins confined to the region in or near the inguinal canal appear to be responsible for most post-surgical persistent or recurrent varicoceles. In our present series, we think that varicocele recurrence was due to small missed spermatic branches with persistent high venous pressure, as all our cases were athletes who do constant heavy lifting and straining. In conventional (non-microscopic) varicocelectomy, postoperative varicocele recurrence is found in 5–20% of men [Citation8].
Conventional varicocelectomy may miss smaller internal spermatic veins that may dilate in the future and cause recurrence [Citation18]. Varicocele recurrence after the inguinal approach is lower than that after the retroperitoneal approach. This is due to accessible external spermatic veins and other perforators in the floor of the inguinal canal. Also, the easy delivery of the cord through the wound facilitates careful dissection of all veins [Citation16,Citation17]. The significant difference between the average number of large veins and average number of smaller veins (P < 0.05) in the same spermatic cord, confirms in our opinion that leaving smaller veins, which are not identified without a microscope, can lead to varicocele recurrence. This probably explains the superiority of microscopic varicocelectomy in minimising varicocele recurrence. Furthermore, our present study showed no significant difference between the average number of vessels including arteries, veins and lymphatics, between the right and left spermatic cords; although there was a tendency for more vessels in the left side cords versus the right side. Currently reported series of laparoscopic varicocelectomy report a recurrence rate of 2.9–4.5% in most recent series, but up to 17% in some [Citation19Citation[20]–Citation21]. The potential complications of laparoscopic varicocelectomy (injury to bowel, vessels or viscera, air embolism, peritonitis) are significantly more serious than those associated with the open techniques. In one series, a microsurgical approach was associated with recurrence rates of <1%. The operating microscope facilitates the identification and ligation of many small spermatic veins, and the preservation of small arteries and lymphatics [Citation22]. This supports our present results with the incidence of varicocele recurrence at 2%, which was clinically not significant. It appears important that all internal and external spermatic veins be ligated to avoid varicocele recurrence [Citation8].
The arterial supply to the testis is derived from three sources: the internal spermatic artery, the deferential (vasal) artery, and the external spermatic (or cremasteric) artery [Citation22]. The internal spermatic artery is intimately associated with the pampiniform plexus of veins. The testicular artery is consistently the largest calibre arterial vessel, with a diameter equal to or greater than the sum of the vasal and cremasteric arteries in >50% of the spermatic cords. This suggests that although the vasal and cremasteric arteries combined contribute significantly to the testicular blood supply, the testicular artery provides most of the blood flow to the human testes. Preservation of the testicular arteries is recommended for optimal testicular blood flow [Citation23]. There is evidence showing deleterious effects of its ligation on germinal epithelium and spermatogenesis from testicular ischaemia in both human and animal models [Citation24].
Internal spermatic arteries at the subinguinal level are covered in a dense complex of veins compared with when they are identified at the inguinal level. This relationship requires greater surgical expertise to separate them. Dissection of this complex places the artery at greater risk of being damaged [Citation25], so every attempt at preservation of encountered arteries should be made. It is worth noting that accidental artery ligation has been reported in 0.9% of cases in a retrospective review of 2102 subinguinal microsurgical cases [Citation26]. However, the incidence of testicular atrophy in patients with accidental ligation was only 5% (one patient in the series) [Citation26].
Subinguinal data show that a solitary artery is identified in 25% of cords, whilst two arteries are identified in 42%, and three or more arteries are identified in 33%. As the internal spermatic artery branches distally in the spermatic cord, the number of arteries identified seems to be dependent on the level of the dissection. In the present study, the average number of spermatic arteries on both sides was 1.3. The increased likelihood of encountering multiple spermatic arteries in the subinguinal approach contributes to the technical difficulty of this approach. Regarding the lymphatics, microscopic varicocelectomy improves identification and preservation of individual lymphatics. Hopps et al. [Citation12] stated that, a mean of 3.2 lymphatics per cord was identified in the subinguinal approach. Our present data demonstrated the average number was ∼ 3 on both sides.
Analysis of the protein concentration of hydrocele fluid indicates that hydrocele formation after varicocelectomy is due to lymphatic obstruction [Citation18]. Moreover, it was reported that impaired lymphatic drainage also impairs testicular function and that postoperative catch up growth is due to interstitial oedema further aggravating fertility. Even testicular histology is changed due to lymphatic stasis [Citation27]. In the present study, there was only one case of hydrocele, with an incidence rate of 0.07%, which was a mild case detected by US. We think that hydroceles result from the accidental ligation of the lymphatics when mistaken for a vein, especially when they overlie a vein. The reported incidence of postoperative hydrocele varies between 3% and 33% (average incidence 7%), thereby representing the most frequent complication [Citation7]. In the retroperitoneal approach, the most common complication after internal spermatic mass ligation is hydrocele, which occurs in up to 25% of cases [Citation28]. Conventional inguinal operations are associated with an incidence of postoperative hydrocele formation varying from 3% to 15% [Citation20,Citation21].
There is still considerable difficulty in discriminating small veins from lymphatic vessels even when using a microscope, which may account for the relatively high percentage of hydroceles. Oswald et al. [Citation29] first described the application of isosulfan blue in varicocele surgery. Lymphatics were clearly stained in 86% after scrotal injection and no cases of hydrocele were evident [Citation24Citation[25]–Citation26]. Finally, use of magnification to identify and preserve lymphatics in microsurgical varicocelectomy can virtually eliminate the risk of hydrocele formation after varicocelectomy [Citation27,Citation7,Citation28]. In our present study, the incidence of hydrocele was 0.07%, which is clinically not significant. The strengths of our present study are a good sized sample and being prospective. The limitation of our study is the lack of the semen analysis outcome data, which we think would have been useful; however, the aim of the present study was to report vascular anatomy details and the relationship with recurrent varicoceles and hydrocele formation. Furthermore, we have reported on outcomes for semen analysis and pregnancy in a previous paper [Citation8]. The effectiveness of microscopic varicocelectomy for semen improvement and pregnancy has been established [Citation30Citation[31]–Citation32].
Conclusion
Microscopic subinguinal varicocelectomy accurately evaluated the detailed vascular anatomy of the spermatic cord, achieving excellent surgical outcome with minimal varicocele recurrence and hydrocele formation. Microscopic subinguinal varicocelectomy should be the ‘gold standard’ for varicocelectomy.
Source of Funding
None.
Conflict of interest
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- G.R.DohleG.M.ColpiT.B.HargreaveG.K.PappA.JungwirthW.WeidnerEAU guidelines on male infertilityEur Urol482005703711
- J.P.JarowI.D.SharlipA.M.BelkerL.I.LipshultzM.SigmanA.J.ThomasBest practice policies for male infertilityJ Urol167200221382144
- C.SivanathanL.J.AbernethyRetrograde embolisation of varicocele in the paediatric age group: a review of 10 years' practiceAnn R Coll Surg8520035051
- L.DubinR.D.AmelarVaricocelectomy: 986 cases in a twelve-year studyUrology101977446449
- S.AlbayrakC.CanK.SaricaExtended vein ligation: a new aspect of the surgical treatment of varicoceleUrol Int511993220224
- P.ChanManagement options of varicocelesIndian J Urol2720116573
- R.D.AmelarEarly and late complications of inguinal varicocelectomyJ Urol1702003366369
- A.M.Al-KandariH.ShabaanH.M.IbrahimY.H.ElshebinyA.A.ShokeirComparison of outcomes of different varicocelectomy techniques: open inguinal, laparoscopic, and subinguinal microscopic varicocelectomy: a randomized clinical trialUrology692007417420
- M.M.WishahiAnatomy of the spermatic venous plexus (pampiniform plexus) in men with and without varicocele: intraoperative venographic studyJ Urol147199212851289
- M.GoldsteinB.R.GilbertA.P.DickerJ.DwoshC.GneccoMicrosurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing techniqueJ Urol148199218081811
- J.L.MarmarT.J.De BenedictisD.PraissThe management of varicoceles by microdissection of the spermatic cord at the external inguinal ringFertil Steril431985583588
- C.V.HoppsM.L.LemerP.N.SchlegelM.GoldsteinIntraoperative varicocele anatomy: a microscopic study of the inguinal versus subinguinal approachJ Urol170200323662370
- B.PajovicN.RadojevicA.DimitrovskiM.RadovicR.RolovicM.VukovicAdvantages of microsurgical varicocelectomy over conventional techniquesEur Rev Med Pharmacol Sci192015532538
- J.L.MarmarThe pathophysiology of varicoceles in the light of current molecular and genetic informationHum Reprod Update72001461472
- R.R.MurrayJrS.E.MitchellS.KadirS.L.KaufmanR.ChangM.L.KinnisonComparison of recurrent varicocele anatomy following surgery and percutaneous balloon occlusionJ Urol1351986286289
- J.SayfanY.G.AdamY.SofferA new entity in varicocele subfertility: the “cremasteric reflux”Fertil Steril3319808890
- S.CayanS.ShavakhabovA.KadiogluTreatment of palpable varicocele in infertile men: a meta-analysis to define the best techniqueJ Androl3020093340
- R.SzaboR.KesslerHydrocele following internal spermatic vein ligation: a retrospective study and review of the literatureJ Urol1321984924925
- M.A.WillJ.SwainM.FodeJ.SonksenG.M.ChristmanD.OhlThe great debate: varicocele treatment and impact on fertilityFertil Steril952011841852
- S.Al-SaidA.Al-NaimiA.Al-AnsariN.YounisA.ShamsodiniK.A-sadiqVaricocelectomy for male infertility: a comparative study of open, laparoscopic and microsurgical approachesJ Urol1802008266270
- I.H.HirschT.A.Abdel-MeguidL.G.GomellaPostsurgical outcomes assessment following varicocele ligation: laparoscopic versus subinguinal approachUrology511998810815
- R.G.HarrisonA.E.BarclayThe distribution of the testicular artery (internal spermatic artery) to the human testisBr J Urol2019485766
- J.D.RamanM.GoldsteinIntraoperative characterization of arterial vasculature in spermatic cordUrology642004561564
- S.J.SilberMicrosurgical aspects of varicoceleFertil Steril311979230232
- E.EnquistB.S.SteinM.SigmanLaparoscopic versus subinguinal varicocelectomy: a comparative studyFertil Steri61199410921096
- P.T.ChanE.J.WrightM.GoldsteinIncidence and postoperative outcomes of accidental ligation of the testicular artery during microsurgical varicocelectomyJ Urol1732005482484
- R.KocvaraJ.DolezalR.HamplC.PovýsilJ.DvorácekM.HillDivision of lymphatic vessels at varicocelectomy leads to testicular oedema and decline in testicular function according to the LH-RH analogue stimulation testEur Urol432003430435
- K.M.FeberE.J.KassVaricocelectomy in adolescent boys: long-term experience with the Palomo procedureJ Urol180Suppl.200816571660
- J.OswaldI.KornerM.RiccabonaThe use of isosulphan blue to identify lymphatic vessels in high retroperitoneal ligation of adolescent varicocele–avoiding postoperative hydroceleBJU Int872001502504
- X.WanH.WangZ.JiMicrosurgical varicocelectomy for clinical varicocele: a review for potential new indicationsAndrologia492017e1282710.1111/and.12827
- A.J.TatemR.E.BranniganThe role of microsurgical varicocelectomy in treating male infertilityTransl Androl Urol62017722729
- Z.LiangJ.GuoH.ZhangC.YangJ.PuH.MeiLymphatic sparing versus lymphatic non-sparing laparoscopic varicocelectomy in children and adolescents: a systematic review and meta-analysisEur J Pediatr Surg212011147153