Abstract
Objective:
To evaluate the effect of oral desmopressin in patients with nocturia associated with benign prostatic hyperplasia (BPH).
Patients and methods:
With a rise of the use of oral desmopressin in the treatment of nocturia in patients with BPH, a systematic review was performed according to the Cochrane systematic reviews guidelines and in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Results:
The literature search yielded 18 studies. The studies were published between 1980 and 2017, and included 3072 patients. Eligible patients were men aged ≥50 years with lower urinary tract symptoms (LUTS) and persistent nocturia. There was a significant 43% reduction in nocturia after using desmopressin alone. Combined α-blockers and desmopressin lead to a decrease in the frequency of night voids by 64.3% compared to 44.6% when using α-blockers only. The first sleep period, significantly increased from 82.1 to 160.0 min and from 83.2 to 123.8 min when using desmopressin + α-blocker and α-blocker only, respectively. The desmopressin dose ranged from the lowest dose (0.05 mg) to the optimum dose (0.4 mg) at bed time. The incidence of hyponatraemia associated with desmopressin use was 4.4–5.7%.
Conclusion:
Low-dose oral desmopressin therapy alone is an effective treatment for nocturia associated with LUTS in patients with BPH. Oral desmopressin combined with α-blockers is well tolerated and beneficial for improving the International Prostate Symptom Score and nocturnal symptoms. All patients should be educated about the mechanism of desmopressin action to avoid treatment discontinuation due to adverse events.
Abbreviations:
- AE, adverse event
- FSP, first sleep period
- ICIQ-N, International Consultation on Incontinence Questionnaire-Nocturia
- ICS, International Continence Society
- NP, nocturnal polyuria
- NPI, Nocturnal Polyuria Index
- PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PVR, post-void residual urine volume
- RCT, randomised controlled trial
Introduction
The adopted definition by the ICS of nocturia [Citation1] is waking up at night once or more to void and being preceded and followed by sleep. Polyuria is defined as a 24-h urine volume of >40 mL/kg body weight, with nocturnal polyuria (NP) defined as a proportion of the 24-h urine voided at night being >20–33%. NP is the most likely cause of persistent nocturia in most patients who are treated for BPH [Citation2].
In patients with BPH, nocturia mostly results from decreased nocturnal bladder capacity [Citation3], high post-void residual urine volume (PVR), and/or detrusor overactivity that decreases functional bladder capacity [Citation4].
Nocturia is a bothersome common storage symptom associated with BPH. However, it does not fully respond to α-blocker therapy, probably due to its multifactorial pathophysiology. It can persist even after effective treatment of BPH [Citation5], therefore desmopressin therapy has been incorporated in to clinical practice [Citation5,Citation6]. The intention of desmopressin therapy, a synthetic derivative of arginine vasopressin (anti-diuretic hormone, ADH), is to substitute lacking endogenous vasopressin.
Desmopressin acetate nasal spray has been approved by the USA Food and Drug Administration (FDA) for the treatment of nocturia due to NP in adults [Citation7]. The orally disintegrating desmopressin tablet avoids ingestion of extra fluids, overcomes swallowing difficulty, and has improved bioavailability compared to the standard tablet [Citation7].
The routine addition of oral desmopressin to α-blocker therapy to improve patients’ bother symptoms is questionable. Should a patient with bothersome nocturia be managed with combined α-blockers and oral desmopressin? We conducted this systematic review to answer this question.
Patients and methods
Search strategy and study selection
A systematic review was performed according to the Cochrane systematic reviews guidelines and in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [Citation8].
A comprehensive search of the medical literature was performed, with no restrictions on the publication language or publication status. The search was conducted to find relevant studies from MEDLINE (1966–2017), EMBASE (1980–2017), Google Scholar, and individual urological journals. The latest search date of all databases was October 2017.
Terms used included: ‘oral desmopressin’, ‘Nocturia’, and ‘benign prostatic hyperplasia’ and ‘LUTS’.
Medical Subject Headings (MeSH) phrases included:
(‘benign prostatic hyperplasia’[MeSH]) AND ‘Nocturia’[MeSH])
((‘benign prostatic hyperplasia’[MeSH]) AND ‘Nocturia’[MeSH]) AND ‘desmopressin’[MeSH])
Inclusion criteria were men being treated for LUTS with nocturia, defined as ≥1 voids/night. We excluded trials of primary or secondary enuresis both in children and adults.
To assess the risk of bias, two reviewers (D.E.T. and O.M.A.) independently identified all potential studies that adhered to the inclusion criteria for full review. The studies selected for inclusion were independently selected by the reviewers. Disagreement between the extracting authors was resolved by consensus or referred to the third author (A.A.S.).
Data extraction and analysis
The objectives were to evaluate the effect of oral desmopressin in nocturia associated with BPH. The following variables were extracted from each study: patient demographics, number of nocturnal voids, duration of first sleep period (FSP), quality of life (QoL), oral desmopressin therapy duration, adverse effects of oral desmopressin, and treatment withdrawal.
Results
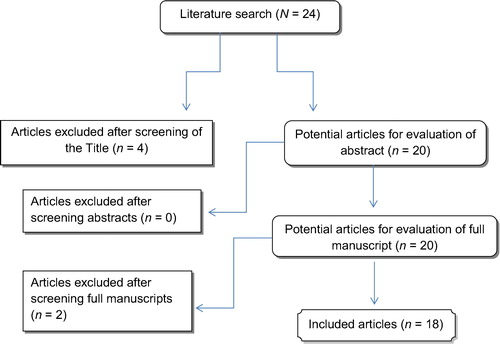
The literature search yielded 18 studies, of which four were excluded due to irrelevance (). All studies were in English, except for one study in Turkish [Citation9]. The titles and abstracts of the studies focused on the effect of desmopressin on nocturia as a separate entity not associated with BPH or LUTS [Citation10], others focused on intranasal desmopressin not the oral formula, also one focused on the incidence of NP in patients with BPH without correlation to desmopressin; hence, the exclusion. All studies reported on the variables indicated in the data extraction section and are listed in .
Table 1 Characteristics of the included studies.
Characteristics of the included studies
The studies were published between 1980 and 2017, and included 3072 patients. Eligible patients were men aged ≥50 years with LUTS and persistent nocturia.
The main outcomes of all the studies was the efficacy of oral desmopressin in reducing nocturia episodes using bladder diaries and improvement in QoL from increasing the duration of undisturbed sleep until first awakening to void.
Effect of desmopressin alone on nocturia
Bae et al. [Citation3] reported in a randomised controlled trial (RCT), including 216 patients, the possible causes of nocturia associated with BPH. Of these patients, there were 158 (76%) with NP, 15 (7.2%) with decreased nocturnal bladder capacity, and 35 (16.8%) with nocturia of both entities.
A 43% significant reduction in nocturia was reported after using desmopressin alone (P < 0.001) [Citation11]. The more severe the nocturia was, the more effective the results with desmopressin, with a 0.756 correlation coefficient between the number of nocturnal voids and the reduction in nocturia after desmopressin treatment.
In another study evaluating the safety and efficacy of long-term desmopressin treatment, the mean night-time frequency decreased by 2.1 voids, and the mean (SD) nocturnal urine voided volume had decreased by 374.2 (261.3) mL at the last follow-up (P < 0.001) [Citation12].
Kuo et al. [Citation13] assessed the outcome of desmopressin based on urodynamic characteristics after 4-weeks treatment. Urodynamic characteristics of the study population were detrusor instability (15 patients) and a cystometric capacity of ≤250 mL (17 patients). There was no significant difference in the success rate relative to the urodynamic characteristics.
Effect of desmopressin vs placebo on nocturia
Several studies compared the effect of desmopressin vs placebo in the control of nocturia [Citation7,Citation10,Citation14-Citation21]. Desmopressin significantly reduced the mean number of nocturnal episodes by 53% and the mean ratio of night/24-h urine volume by 39% (P = 0.034). The undisturbed sleep hours were significantly increased by 74%. Moreover, patients were more willing to continue on desmopressin in comparison with placebo [Citation17]. Desmopressin was significantly superior to placebo in terms of the Nocturnal Polyuria Index (NPI; P = 0.001), International Consultation on Incontinence Questionnaire-Nocturia (ICIQ-N) (P = 0.001), and total IPSS (P = 0.041) [Citation17].
Effect of desmopressin combined with α-blockers on nocturia
There were some RCTs comparing the effect of combined tamsulosin and oral desmopressin vs tamsulosin alone [Citation3,Citation22]. There was a decrease in the night voiding frequency by 64.3% in the desmopressin + tamsulosin group compared to 44.6% in tamsulosin-only group. The change in IPSS was more significant in the α-blocker group. The FSP significantly increased from 82.1 to 160.0 min and from 83.2 to 123.8 min in desmopressin + tamsulosin and tamsulosin-only groups, respectively.
Kim et al. [Citation17,Citation23] studied the effectiveness of silodosin alone or as an add-on therapy to desmopressin. There was a significant improvement in the IPSS and QoL (P = 0.001 and P < 0.001, respectively). There was an improvement in the Leeds Sleep Evaluation Questionnaire (LSEQ) score from 64.36 to 70.43 (P = 0.039). Moreover, there was also a reduction in the NPI from 0.4005 to 0.3573 (P = 0.027)
Some studies [Citation9] evaluated the use of combined desmopressin and alfuzosin with alfuzosin monotherapy. There was a 36.11% reduction in the mean nocturia number in patients receiving combined therapy, which was significantly higher than in patients receiving alfuzosin only (25.71%).
Desmopressin significantly decreased the number of nocturnal voids from a baseline mean of 7.0 to 5.7 episodes for 3 days at the 24-week visit (P = 0.03) [Citation3,Citation9].
One study [Citation24] evaluated the value of anticholinergic or antidiuretic agent order, either to start with anticholinergic or antidiuretic, as an add-on therapy in men previously treated with an α-blocker for LUTS in a randomised controlled design. Both anticholinergic and desmopressin resulted in a significant decrease in nocturnal urine volume, nocturia episodes, overactive bladder symptom score, urgency episodes, and nocturnal bladder capacity index. Nocturia including LUTS improved with the use of anticholinergic or antidiuretic agents as an add-on therapy to an α-blocker, whilst, there was no benefit of medication order.
Berges et al. [Citation14] reported a significant improvement in both the mean IPSS-QoL (by 43%) and mean ICIQ-N nocturia problem question (by 53%) on using desmopressin only, whilst concomitant α-blocker had no effect on desmopressin efficacy.
Desmopressin dose
The desmopressin dose ranged from the lowest dose (0.05 mg) to an optimum dose (0.4 mg) at bed time [Citation16,Citation18,Citation19]. The dose was titrated according to either, the patient response based on number of nocturnal voids and degree of bother from the symptoms [Citation12,Citation18], increased weekly [Citation16], or during a 3-week interval [Citation21] until acquiring the desired response. Weiss et al. [Citation10] reported that 50 and 75 µg desmopressin had a similar efficacy and they recommended a 50 µg desmopressin dose in order to reduce the incidence of hyponatraemia.
One study [Citation10] assessed the response to desmopressin based on dosing. The 50 and 75 µg doses significantly reduced the number of nocturnal voids (−0.37, P < 0.001 and −0.41, P = 0.003, respectively) compared with placebo over 3 months. Desmopressin 50 and 75 µg increased the time to first void from baseline by ∼40 min compared to placebo (P = 0.006 and P = 0.003, respectively). The response to desmopressin was seen after 1 week of treatment and was sustained.
Adverse effects
The overall incidence of adverse events (AEs) ranged from 2.2% [Citation14] to 6.61% [Citation16]. Some patients discontinued their treatment due to diarrhoea [Citation14] or hyponatraemia. Other patients did not discontinue desmopressin due to less severe symptoms [Citation16].
The most reported AE was hyponatraemia. Other AEs included: headache, dizziness, nausea, oliguria, diarrhoea, oliguria, and incontinence [Citation16].
Desmopressin therapy gradually decreases serum sodium. Serum sodium should be assessed carefully, at least at 1 week after treatment. Some studies noted that there were no significant hyponatraemia associated with desmopressin use [Citation17,Citation20,Citation22,Citation25].
The incidence of hyponatraemia with desmopressin treatment ranges from 4.4% [Citation12] to 5.7% [Citation22]. In a study, involving in >250 000 patients, Delfanian and Zawada [Citation26] reported certain potential risks for occurrence of hyponatraemia after desmopressin administration. These included surgery stress, hepatic disease, increased desmopressin dose, and excessive fluid intake. They concluded that hyponatraemia may be prevented by close monitoring of serum electrolytes and avoidance of low sodium solutions.
Chen et al. [Citation16] assessed the serum sodium level at 1, 4, and 12 weeks after initiation of desmopressin therapy. The mean (SD) decrease in the serum sodium levels was 3.89 (1.22) mmol/L (P < 0.001) in the non-NP group and 4.69 (3.5) mmol/L (P < 0.001) in the NP group.
Lower desmopressin dose and gender-specific dosing is of value to reduce clinically significant hyponatraemia. As in one study there were reductions in serum sodium to <125 mmol/L in six women (taking >25 µg desmopressin) and two men (aged 67 and 82 years) taking 100 µg [Citation7].
Discussion
Nocturia is defined as waking up from sleep at night one or more times to void, each void being preceded and followed by sleep [Citation1]. Based on the degree of bother, the number of night voids that defines clinically significant nocturia is ≥2 voids/night [Citation27]. Only 5% of men and 9.2% of women present with nocturia as a single symptom, whereas nocturia concomitantly appears with other storage and/or voiding LUTS in 20.5% of men and 17.4% of women [Citation28].
In patients with BPH, being the most common cause of nocturia, nocturia mostly results from a high PVR and/or detrusor overactivity, which decreases functional bladder capacity [Citation4]. Also nocturia may be due to NP or decreased nocturnal bladder capacity [Citation3].
Nocturia cannot be explained alone based on BPH, but can be consecutive to polyuria, polydipsia, diabetes mellitus, cardiac failure, neurogenic bladder, reduced bladder capacity, insomnia, or psychiatric problems [Citation1,Citation4,Citation29]. To reduce nocturia, action should be directed at BOO, bladder sensitivity by anticholinergics, somnolence by hypnotic drugs, or urinary volume by antidiuretics [Citation30].
The serum concentration of the antidiuretic agent vasopressin increases during the night leading to decreasing urine secretion. Secretion of this hormone diminishes with age, when the renal response to antidiuretic agents decreases and the reduction of the total amount of nephrons limit the renal response to the hormone [Citation30].
Behavioural modification, in the form of instructing patients to avoid nightly known diuretic beverages, may be a possible contributing factor. Also, it is hypothesised that the combined rise in functional voiding capacity and improvement in urinary flow rates can increase the mean urine volume/void, with resultant decreases in daytime and nocturnal frequencies [Citation31].
Desmopressin acetate (Minirin®) is a synthetic analogue of arginine vasopressin, with similar antidiuretic action but without its pressor effects, which is effective in the treatment of polyuric states, such as primary nocturnal enuresis and central diabetes insipidus [Citation32,Citation33]. Desmopressin has a Level 1, Grade A recommendation from the International Consultation on Incontinence and European Association of Urology for the treatment of nocturia associated with polyuria [Citation34–Citation36]. The recent desmopressin formula, is an orally disintegrating tablet that overcomes swallowing difficulty, avoids ingestion of extra fluids, and has improved bioavailability compared to the standard tablet [Citation7].
Desmopressin alone significantly decreased the number of nocturnal voids from a baseline mean of 7.0 to 5.7 episodes for 3 days at the 24-week visit [Citation3,Citation9].
In comparison with placebo, desmopressin significantly reduced the mean number of nocturnal voids by 53% and the mean ratio of night/24-h urine volume by 39%. The hours of undisturbed sleep significantly increased by 74% allowing undisturbed sleep for ≥4 h [Citation10,Citation14,Citation17,Citation20,Citation37].
The frequencies of night voids decreased by 64.3% with desmopressin + tamsulosin compared to 44.6% with tamsulosin-only treatment [Citation3,Citation22,Citation38]. The FSP, significantly increased from 82.1 to 160.0 min and from 83.2 to 123.8 min with desmopressin + tamsulosin combined treatment and tamsulosin-only treatment, respectively [Citation3,Citation22,Citation38]. Although the combined tamsulosin and desmopressin treatment was more effective in treating nocturia, the change in IPSS was more significant in the α-blocker-only group.
Combined desmopressin and alfuzosin combined therapy resulted in a 36% reduction in the mean nocturia number in comparison to 25% with alfuzosin-only therapy [Citation9].
The desmopressin dose ranged from the lowest dose (0.05 mg) to the optimum dose (0.4 mg) at bed time [Citation16,Citation18,Citation19]. The dose was titrated according to either, the patient response based on the number of nocturnal voids and degree of bother by symptoms [Citation18], or increased at weekly intervals until the desired response was attained [Citation16]. Treatment with 50 µg desmopressin, the minimum effective dose, provided sustained improvement of nocturia and has fewer incidences of hyponatraemia (serum sodium ≤ 125 mmol/L). The 50 and 75 µg desmopressin have similar efficacy [Citation10].
Based on dosing [Citation10], the 50 and 75 µg doses significantly reduced the number of nocturnal voids (−0.37, P < 0.001 and −0.41, P = 0.003, respectively) compared with placebo at 3 months. Desmopressin 50 and 75 µg increased the time to first void from baseline by ∼40 min compared to placebo (P = 0.006 and P = 0.003, respectively). The response to desmopressin was seen by 1 week of treatment and was sustained.
The overall incidence of AEs ranged from 2.2% [Citation14] to 6.61% [Citation16]. The most reported AE was hyponatraemia. Other AEs included: headache, dizziness, nausea, oliguria, diarrhoea, and incontinence [Citation16]. Some patients discontinued treatment due to diarrhoea [Citation14] or hyponatraemia.
The incidence of hyponatraemia with desmopressin treatment ranges from 4.4% [Citation12] to 5.7% [Citation22]. In a study, involving in >250 000 patients, Delfanian and Zawada [Citation26] reported certain potential risks for the occurrence of hyponatraemia after desmopressin administration. These included surgery stress, hepatic disease, increased desmopressin dose, and excessive fluid intake. They concluded that hyponatraemia may be prevented by close monitoring of serum electrolytes and avoidance of low sodium solutions.
Serum sodium should be assessed carefully, at least at 1 week after treatment, However, some studies noted that there were no significant hyponatraemia associated with desmopressin use [Citation17,Citation20,Citation22,Citation25].
Lower desmopressin dose and gender-specific dosing is of value to reduce clinically significant hyponatraemia. As in one study there were reductions in serum sodium to <125 mmol/L in six women (taking >25 µg desmopressin) and two men (aged 67 and 82 years) taking 100 µg [Citation7].
Conclusion
Low-dose oral desmopressin therapy alone is an effective treatment of nocturia associated with LUTS in patients with BPH. Oral desmopressin added to α-blockers is well tolerated and beneficial for improving the IPSS and nocturnal symptoms. All patients should be educated about the mechanism of desmopressin action in order to avoid treatment discontinuation due to AEs.
Acknowledgement
None.
Support and grants
None.
Conflicts of interest
None.
Notes
Peer review under responsibility of Arab Association of Urology.
References
- P.van KerrebroeckP.AbramsD.ChaikinJ.DonovanD.FondaS.Jacksonet al.The standardisation of terminology in nocturia: report from the standardisation sub-committee of the International Continence SocietyNeurourol Urodyn212002179183
- H.F.YoongM.B.SundaramZ.AidaPrevalence of nocturnal polyuria in patients with benign prostatic hyperplasiaMed J Malaysia602005294296
- W.J.BaeJ.H.BaeS.W.KimB.H.ChungJ.H.KimC.S.Kimet al.Desmopressin add-on therapy for refractory nocturia in men receiving α-blockers for lower urinary tract symptomsJ Urol1902013180186
- K.YoshimuraH.OharaK.IchiokaN.TeradaY.MatsuiA.Teraiet al.Nocturia and benign prostatic hyperplasiaUrology612003786790
- J.P.WeissJ.G.BlaivasD.S.StemberM.M.BrooksNocturia in adults: etiology and classificationNeurourol Urodyn171998467472
- A.ZiadaM.RosenblumE.D.CrawfordBenign prostatic hyperplasia: an overviewUrology53Suppl. 3a199916
- J.P.WeissN.R.ZinnerB.M.KleinJ.P.NørgaardDesmopressin orally disintegrating tablet effectively reduces nocturia: results of a randomized, double-blind, placebo-controlled trialNeurourol Urodyn312012441447
- A.LiberatiD.G.AltmanJ.TetzlaffC.MulrowP.C.GøtzscheJ.P.Ioannidiset al.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaborationBMJ3392009 b270010.1136/bmj.b2700
- O.KocaM.O.KeleşM.GüneşM.ÖztürkM.AkyüzM.I.KaramanDesmopressin in the treatment of nocturia with BPHTurk J Urol3820122931
- J.P.WeissS.HerschornC.D.AlbeiE.A.van der MeulenEfficacy and safety of low dose desmopressin orally disintegrating tablet in men with nocturia: results of a multicenter, randomized, double-blind, placebo controlled, parallel group studyJ Urol1902013965972
- D.R.HoW.Y.LinC.F.WuJ.J.SheeY.C.HuangC.S.ChenClinical observations of the effect of antidiuretic hormone on nocturia in elderly menBJU Int96200513101313
- M.SongB.S.HongJ.Y.ChunJ.Y.HanM.S.ChooSafety and efficacy of desmopressin for the treatment of nocturia in elderly patients: a cohort studyInt Urol Nephrol46201414951499
- H.C.KuoEfficacy of desmopressin in treatment of refractory nocturia in patients older than 65 yearsUrology592002485489
- R.BergesK.HöfnerM.GedamkeM.OelkeImpact of desmopressin on nocturia due to nocturnal polyuria in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH)World J Urol32201411631170
- A.CannonP.G.CarterA.A.McConnellP.AbramsDesmopressin in the treatment of nocturnal polyuria in the maleBJU Int8419992024
- S.L.ChenY.H.HuangT.W.HungY.C.OuComparison of nocturia response to desmopressin treatment in elderly men with and without nocturnal polyuria in real-life practiceInt J Clin Pract702016372379
- J.C.KimK.J.ChoJ.G.LeeJ.T.SeoD.Y.KimS.J.Ohet al.Efficacy and safety of desmopressin add-on therapy for men with persistent nocturia on alpha-blocker monotherapy for lower urinary tract symptoms: a randomized, double-blind, placebo controlled studyJ Urol1972017459464
- A.MattiassonP.AbramsP.Van KerrebroeckS.WalterJ.WeissEfficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in menBJU Int892002855862
- B.RezakhanihaN.ArianpourS.SiroosbakhatEfficacy of desmopressin in treatment of nocturia in elderly menJ Res Med Sci162011516523
- C.J.WangY.N.LinS.W.HuangC.H.ChangLow dose oral desmopressin for nocturnal polyuria in patients with benign prostatic hyperplasia: a double-blind, placebo controlled, randomized studyJ Urol1852011219223
- P.van KerrebroeckM.RezapourA.CortesseJ.ThüroffA.RiisJ.P.NørgaardDesmopressin in the treatment of nocturia: a double-blind, placebo-controlled studyEur Urol522007221229
- A.F.AhmedA.MaaroufE.ShalabyA.H.GabrA.ShahinA.GhobishThe impact of adding low-dose oral desmopressin therapy to tamsulosin therapy for treatment of nocturia owing to benign prostatic hyperplasiaWorld J Urol332015649657
- Y.W.KimJ.ParkH.ChungH.W.KimH.J.KimJ.H.Junget al.The effectiveness of silodosin for nocturnal polyuria in elderly men with benign prostatic hyperplasia: a multicenter studyInt Neurourol J192015190196
- Y.S.ShinL.T.ZhangC.ZhaoY.G.KimJ.K.ParkTwelve-week, prospective, open-label, randomized trial on the effects of an anticholinergic agent or antidiuretic agent as add-on therapy to an alpha-blocker for lower urinary tract symptomsClin Interv Aging9201410211030
- M.B.ChancellorA.AtanD.A.RivasT.WatanabeH.L.TaiH.KumonBeneficial effect of intranasal desmopressin for men with benign prostatic hyperplasia and nocturia: preliminary resultsTech Urol51999191194
- K.DelfanianE.T.ZawadaJr.DDAVP-associated hyponatremiaS D J Med542001255256
- K.A.TikkinenT.M.Johnson2ndT.L.TammelaH.SintonenJ.HaukkaH.Huhtalaet al.Nocturia frequency, bother, and quality of life: How often is too often? A population-based study in FinlandEur Urol572010488496
- M.OelkeP.AndersonR.WoodT.Holm-LarsenNocturia is often inadequately assessed, diagnosed and treated by physicians: results of an observational, real-life practice database containing 8659 European and US-American patientsInt J Clin Pract702016940949
- F.MontorsiD.MercadanteDiagnosis of BPH and treatment of LUTS among GPs: a European surveyInt J Clin Pract672013114119
- R.AsplundH.AbergDiurnal variation in the levels of antidiuretic hormone in the elderlyJ Intern Med2291991131134
- M.YoshidaA.InadomeK.MasunagaT.NagataT.YoshiyasuEffectiveness of tamsulosin hydrochloride and its mechanism in improving nocturia associated with lower urinary tract symptoms/benign prostatic hyperplasiaNeurourol Urodyn29201012761281
- H.VilhardtBasic pharmacology of desmopressinDrug Invest2Suppl. 5199028
- A.F.AhmedM.M.AminM.M.AliE.A.ShalabyEfficacy of an enuresis alarm, desmopressin, and combination therapy in the treatment of Saudi children with primary monosymptomatic nocturnal enuresisKorean J Urol542013783790
- J.W.ThüroffP.AbramsK.E.AnderssonW.ArtibaniC.R.ChappleM.J.Drakeet al.EAU guidelines on urinary incontinenceEur Urol592011387400
- P.AbramsK.E.AnderssonL.BirderL.BrubakerL.CardozoC.Chappleet al.Fourth international consultation on incontinence recommendations of the international scientific committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinenceNeurourol Urodyn292010213240
- M.OelkeA.BachmannA.DescazeaudM.EmbertonS.GravasM.C.Michelet al.Guidelines on the Management of Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO)2012European Association of Urology Available at: https://pdfs.semanticscholar.org/f332/3556c2548714f6fb03f00d697f2ea19f5c77.pdf. Accessed June 2018
- W.MånssonT.SundinB.GullbergEvaluation of a synthetic vasopressin analogue for treatment of nocturia in benign prostatic hypertrophy. A double-blind studyScand J Urol Nephrol141980139141
- C.CeylanT.CeylanO.G.DoluogluS.YukselK.AgrasComparing the effectiveness of intranasal desmopressin and doxazosin in men with nocturia: a pilot randomized clinical trialUrol J102013993998