?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The Randić index is one of the classical graph-based molecular structure descriptors that found countless applications in chemistry and pharmacology. The mathematical background of this index is also well elaborated. We now point out a hitherto unnoticed feature of
, namely its connection with the degree-based information content of a (molecular) graph. This connection is based on the linear correlation between
and the logarithm of the multiplicative version of the Randić index.
1 Introduction
The Randić index (also known under the name connectivity index) is a much investigated degree-based topological index [1–3Citation[1]Citation[2]Citation[3]]. It was invented in 1976 by Milan Randić [Citation1] and is defined as (1.1)
(1.1) where the notation used is defined below. Among the several hundred presently existing graph-based molecular structure descriptors [Citation4,Citation5], the Randić index is certainly the most widely applied in chemistry and pharmacology, in particular for designing quantitative structure–property and structure–activity relations. Details of these applications can be found in the books [Citation3,Citation6,Citation7] and the surveys written by Randić himself [Citation8,Citation9].
After a delay longer than 20 years, mathematicians recognized that the graph invariant possesses a wealth of interesting and non-trivial mathematical properties [Citation10,Citation11]. This eventually resulted in extensive mathematical research along these lines and countless published papers; for details see [Citation12,Citation13] and the references quoted therein. Especially remarkable is the discovery of an algebraic connection between the Randić index and the normalized Laplacian matrix [14–16Citation[14]Citation[15]Citation[16]].
In the present paper, we point out a further, hitherto unnoticed, feature of the Randić index, namely its connection with the information content of the underlying molecular graph.
In order to achieve this goal, we need some preparations.
2 Graph-theoretical definitions and results
Let be a (molecular) graph [Citation17,Citation18] and let
and
be its vertex and edge sets, respectively. This graph is assumed to possess n vertices and m edges. If u and v are two adjacent vertices of the graph G, then the edge connecting them will be denoted by uv.
The degree of the vertex v is the number of first neighbors of this vertex. According to a well known result of graph theory,
(2.1)
(2.1)
The Randić index of the (molecular) graph G is defined via Eq. (Equation1.1
(1.1)
(1.1) ). It is one of the several dozens of vertex-degree-based molecular structure descriptors of the general form
that have been and are being considered in contemporary mathematical chemistry and chemometrics; for details see [Citation2,Citation4,Citation5].
Some time ago, Todeschini and Consonni [Citation19] proposed to extend the area of degree-based molecular structure-descriptors by examining multiplicative topological indices of the form (2.2)
(2.2) where
indicates the product of the terms
. The first such topological index studied was the multiplicative version of the Wiener index [20–22Citation[20]Citation[21]Citation[22]]. Recently, the multiplicative Zagreb indices attracted much attention [23–26Citation[23]Citation[24]Citation[25]Citation[26]]. To the present authors’ best knowledge, the multiplicative Randić index has not been considered until now.
Bearing in mind Eqs. (Equation1.1(1.1)
(1.1) ) and (Equation2.2
(2.2)
(2.2) ), we may define the multiplicative Randić index as
(2.3)
(2.3)
Since any vertex is adjacent to exactly
other vertices, in the product on the right-hand side of Eq. (Equation2.3
(2.3)
(2.3) ), each term
appears
times. This yields:
Theorem 2.1
i.e., (2.4)
(2.4)
At this point, we have to recall that the information content of a system for which a probability distribution holds, is equal to [Citation27,Citation28]
(2.5)
(2.5)
As usual, in Eq. (Equation2.5(2.5)
(2.5) ), the conditions
and
are assumed to be obeyed. Applying formula (Equation2.5
(2.5)
(2.5) ) to molecules and molecular graphs, it was possible to conceive a variety of chemically relevant information contents of chemical compounds [Citation28]. In view of the obvious formal analogy between Eqs. (Equation2.4
(2.4)
(2.4) ) and (Equation2.5
(2.5)
(2.5) ), we may think of a vertex-degree-based information content of the (molecular) graph G.
In order to obey the basic requirements behind formula (Equation2.5(2.5)
(2.5) ), introduce the auxiliary quantities
which, according to Eq. (Equation2.1
(2.1)
(2.1) ), satisfy the necessary conditions
and
. Then the vertex-degree-based information content of the graph G is
Eq. (Equation2.4
(2.4)
(2.4) ) can now be rewritten as:
which implies our final result:
Theorem 2.2
Let be a graph with
edges. The vertex-degree-based information content of
and the multiplicative Randić index
are related as:
(2.6)
(2.6)
3 The Randić index connection
Theorem 2.2 is a straightforward consequence of the definition (Equation2.3(2.3)
(2.3) ) of the multiplicative Randić index. It, however, does not connect the ordinary Randić index, Eq. (Equation1.1
(1.1)
(1.1) ), with any information content of the (molecular) graph G. In order to arrive at such a connection, we have to establish a relation between
and
.
Within the earlier studies of the multiplicative Wiener index [Citation20,Citation21], it was discovered that there exists an excellent linear correlation between the logarithm of the multiplicative Wiener index and the ordinary Wiener index. Such correlations were found to hold for various classes of molecular graphs of isomeric compounds. Bearing this in mind, the obvious property that had to be checked is whether analogous correlations exist also between and
. By means of an extensive numerical work we confirmed that, indeed, such a correlation does exist, i.e., that within classes of molecular graphs with fixed values of the parameters n and m, the equality
(3.1)
(3.1) holds as a fairly accurate approximation, with a and b being empirically determined constants (depending on n and m). If relation (Equation3.1
(3.1)
(3.1) ) is substituted back into (Equation2.6
(2.6)
(2.6) ), then the information content
becomes dependent on the Randić index in a simple, linear manner:
(3.2)
(3.2) where A and B are empirically determined constants, depending on n and m. Details of our numerical tests of relation (Equation3.2
(3.2)
(3.2) ) are described in the subsequent section.
4 Numerical work
The quality of the approximation given by Eq. (Equation3.2(3.2)
(3.2) ) was tested on chemical trees from 8 to 20 vertices, as well as on unicyclic, bicyclic, …, pentacyclic 10-vertex connected chemical graphs. These data were generated using the Open Molecule Generator [Citation29]. It creates data as sdf-files. An in-house Python program was developed for the calculation of vertex-degree-based information content of a graph, Randić index and statistical parameters that show an extent of correlation between these topological indices. The Open Babel module [Citation30] has been used for reading sdf-files and generating adjacency matrices from alkanes.
The obtained statistical parameters for chemical trees are collected in . Correlation coefficient is approximately 0.95 and its value decreases by increasing the order of chemical trees. Standard error of estimate is decreasing in the same manner.
Table 1 Slope, intercept, correlation coefficient and standard error of the estimate (Equation3.2 (3.2)
(3.2)  (3.2) ) for chemical trees from 8 to 20 vertices.
(3.2) ) for chemical trees from 8 to 20 vertices.
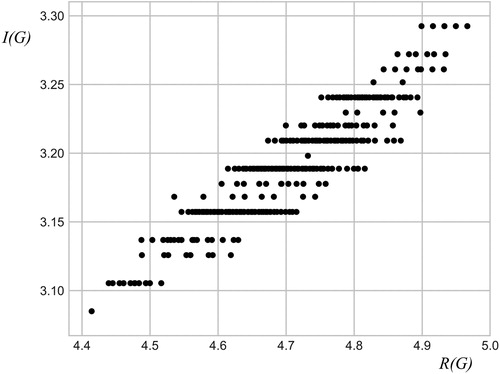
An illustration of linear correlation between I (G) and R (G) in the case of chemical trees is given in .
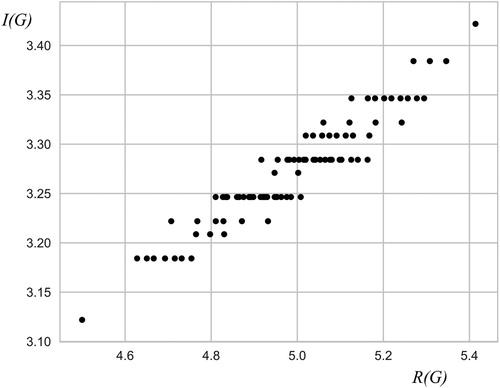
Further investigation of the dependence between I (G) and R (G) was extended also to connected chemical graphs containing cycles. In this study, 10-vertex chemical graphs having up to 5 cycles were considered. Linear correlation between the vertex-degree-based information content of a graph and Randić index has also been confirmed. The correlation coefficient is approximately 0.94. The standard error of the estimate (Equation3.2(3.2)
(3.2) ) is decreasing with the number of cycles. The results obtained are summarized in .
Table 2 Slope, intercept, correlation coefficient and standard error of the estimate (Equation3.2 (3.2)
(3.2)  (3.2) ) for ten-vertex connected chemical graph having up to 5 cycles.
(3.2) ) for ten-vertex connected chemical graph having up to 5 cycles.
A pictorial example of the quality of linear correlation between I (G) and R (G) in the case of 10-vertex cyclic connected chemical graphs is given in .
5 Discussion and concluding remarks
Numerical investigation of linear dependence between vertex-degree-based information content of a graph and Randić index has been demonstrated in all conducted numerical experiments. The quality of the correlation is quantified by Pearson correlation coefficient and standard error of an estimate. Results presented in and indicate a reasonably good linear correlation.
However, further inspection of the plotted data (see and ) reveals data-clustering onto several horizontal lines. This indicates that I (G) (contrary to R (G)) carries information only on degree distribution in graphs and not on their mutual interplay. The insensitivity of vertex-degree-based information of a graph on subtle structural differences among graphs reduces its application potential in chemistry.
Notes
Peer review under responsibility of Kalasalingam University.
References
- Randić M. On characterization of molecular branching J. Am. Chem. Soc. 97 1975 6609 6615
- Gutman I. Degree–based topological indices Croat. Chem. Acta 86 2013 351 361
- Randić M. Novič M. Plavšić D. Solved and Unsolved Problems in Structural Chemistry 2016 CRC Press Boca Raton
- Todeschini R. Consonni V. Handbook of Molecular Descriptors 2000 Wiley–VCH Weinheim
- Todeschini R. Consonni V. Molecular Descriptors for Chemoinformatics 2009 Wiley–VCH Weinheim
- Kier L.B. Hall L.H. Molecular Connectivity in Chemistry and Drug Research 1976 Academic Press New York
- Kier L.B. Hall L.H. Molecular Connectivity in Structure–Activity Analysis 1986 Wiley New York
- Randić M. The connectivity index 25 years after J. Mol. Graph. Model. 20 2001 19 35
- Randić M. On history of the Randić index and emerging hostility toward chemical graph theory MATCH Commun. Math. Comput. Chem. 59 2008 5 124
- Bollobás B. Erdős P. Graphs of extremal weights Ars Combin. 50 1998 225 233
- Bollobás B. Erdős P. Sarkar A. Extremal graphs for weights Discrete Math. 200 1999 5 19
- Li X. Gutman I. Mathematical Aspects of Randić Type Molecular Structure Descriptors 2006 Univ. Kragujevac Kragujevac
- Li X. Shi Y. A survey on the Randić index MATCH Commun. Math. Comput. Chem. 59 2008 127 156
- Cavers M. Fallat S. Kirkland S. On the normalized Laplacian energy and general Randić index R−1 of graphs Linear Algebra Appl. 433 2010 172 190
- Das K.C. Sun S. Gutman I. Normalized Laplacian eigenvalues and Randić energy of graphs MATCH Commun. Math. Comput. Chem. 77 2017 45 59
- Das K.C. Sun S. Extremal graphs for Randić energy MATCH Commun. Math. Comput. Chem. 77 2017 77 84
- Trinajstić N. Chemical Graph Theory 1983 CRC Press Boca Raton
- Gutman I. Polansky O.E. Mathematical Concepts in Organic Chemistry 1986 Springer Berlin
- Todeschini R. Consonni V. New local vertex invariants and molecular descriptors based on functions of the vertex degrees MATCH Commun. Math. Comput. Chem. 64 2010 359 372
- Gutman I. Linert W. Lukovits I. Ž. Tomović . The multiplicative version of the Wiener index J. Chem. Inf. Comput. Sci. 40 2000 113 116
- Gutman I. Linert W. Lukovits I. Tomović Ž. On the multiplicative Wiener index and its possible chemical applications Monatsh. Chem. 131 2000 421 427
- Das K.C. Gutman I. On Wiener and multiplicative Wiener indices of graphs Discrete Appl. Math. 206 2016 9 14
- Božović V. Kovijanić Vukićević Ž. Popivoda G. Chemical trees with extreme values of a few types of multiplicative Zagreb indices MATCH Commun. Math. Comput. Chem. 76 2016 207 220
- Eliasi M. Ghalavand A. Ordering of trees by multiplicative second Zagreb index Trans. Combin. 5 2016 49 55
- Kazemi R. Note on the multiplicative Zagreb indices Discrete Appl. Math. 198 2016 147 154
- Kazemi R. On the multiplicative Zagreb indices of bucket recursive trees Iranian J. Math. Chem. 8 2017 37 45
- Shannon C.E. A mathematical theory of communication Bell Syst. Tech. J. 27 379–423 1948 623 656
- Bonchev D. Information Theoretic Indices for Characterization of Chemical Structures 1983 Research Studies Press Chichester
- Peironcely J.E. Rojas-Chertó M. Fichera D. Reijmers T. Coulier L. Faulon J.L. Hankemeier T. OMG: Open molecule generator J. Cheminformatics 4 2012 #21
- O’Boyle N.M. Banck M. James C.A. Morley C. Vandermeersch T. Hutchison G.R. Open Babel: An open chemical toolbox J. Cheminformatics 3 2011 #33