Abstract
Background
Thoracic paravertebral block (TPVB) is an effective intraoperative and postoperative technique for surgical anaesthesia and analgesia for breast surgery. It offers a long-lasting effective analgesia without increases in side effects, with a significant decrease in anaesthetic and analgesic consumption, and with a high degree of patient satisfaction and shorter recovery time. In this study, TPVB was done by using a nerve stimulator to measure the depth of needle insertion by eliciting intercostal muscle contraction, and a catheter was introduced preoperatively to allow for repeated injections and to maintain analgesia postoperatively.
Methods
Two groups of patients undergoing unilateral cancer breast surgery (each 20 patients) were randomly assigned to the study; a study group (PVB) and a control (C) group. The study started by preoperative application of an epidural catheter by using the nerve stimulator at the fourth thoracic paravertebral space in the study group and injection of local anaesthetic started preoperatively. General anaesthesia was started for the two groups. Total intraoperative fentanyl and postoperative morphine consumption, and pain intensity at rest and with arm movement were recorded, together with recording of any undesirable side effects for 24 h.
Results
There were statistically highly significant decreases in intraoperative fentanyl consumption and postoperative morphine consumption in the PVB group than the C group. There were statistically significant decreases in the VAS in the PVB group than the C group both at rest and with shoulder movement. The incidence of adverse events was very low in both groups.
Conclusion
Continuous TPVB provides effective pain relief, significant opioid sparing, and also less painful restricted movement of the shoulder, with few side effects after breast cancer surgery. Thoracic paravertebral somatic nerve block may be an alternative to general anaesthesia for major unilateral breast surgery with heavy sedation or to be combined with light general anaesthesia.
1 Introduction
Thoracic paravertebral block (TPVB) is becoming increasingly popular, especially as an anaesthetic adjunct for breast surgery. The best anaesthetic technique for breast cancer surgery allows for good intraoperative and postoperative analgesia with early hospital discharge. Breast cancer surgery with axillary dissection is usually performed under general anaesthesia. TPVB is an effective intraoperative and postoperative technique for surgical anaesthesia and analgesia for breast surgery. It offers a long-lasting effective analgesia without increases in side effects, with a significant decrease in anaesthetic and analgesic consumption, and with a high degree of patient satisfaction and shorter recovery time [Citation1–Citation4].
Thoracic paravertebral block (TPVB) is the technique of injecting local anaesthetic adjacent to the thoracic vertebra close to where the spinal nerves emerge from the intervertebral foramina. This results in ipsilateral somatic and sympathetic nerve blockade in multiple contiguous thoracic dermatomes above and below the site of injection [Citation2].
In this study, TPVB was done by using a nerve stimulator to measure the depth of needle insertion by eliciting intercostal muscle contraction, and a catheter was introduced preoperatively to allow for repeated injections and to maintain analgesia postoperatively. TPVB was evaluated for its intraoperative anaesthesia and analgesia and postoperative analgesic effect in patients undergoing breast cancer surgery with recording of the intraoperative and postoperative narcotic consumption and any untoward side effects.
2 Patients and methods
The study was obtained after our institutional review board, and after giving written, informed consent from the participating patients in this randomized, prospective, single-blinded study. Forty patients, classified as ASA physical status I–II, aged 18–60 years, were scheduled for unilateral breast surgery; either wide local excisions (at least one breast quadrant) with axillary dissection, or modified radical mastectomies.
Patients were randomly divided into two groups, paravertebral block group (PVB group) in which patients were candidates for general anaesthesia after thoracic paravertebral catheter application and local anaesthetic injection, and the control C group were candidates for general anaesthesia. Patient exclusion criteria included patient refusal, coagulopathy, infection at the site of block placement, chest deformity, obstructive or restrictive lung disease, morbid obesity (twice the ideal body weight or >130 kg), and sensitivity to local anaesthetics.
The study started in the preoperative holding area. Patients were instructed in the use of the 100 mm visual analogue score (VAS): a pain VAS (0 = no pain to 100 = worst imaginable pain). Sedation was achieved for patients for TPVB with intravenous midazolam 1–5 mg, while the patients were in the lateral left decubitus position. Blood pressure, ECG, and oxygen saturation were monitored, and an oxygen face mask 3 L min−1 was applied. The injection site was marked 2.5 cm laterally to the midline determined by manual palpation (spinous process of T4) ipsilateral to the operative breast. After aseptic preparation of the skin, the injection site was infiltrated with lidocaine 1% 1–2 ml. A 10 cm 21 G insulated needle (Stimuplex, B. Braun, Melsungen, Germany), attached to a nerve stimulator (initial stimulating current: 5 mA, 1 Hz, 9 V; Stimuplex, B. Braun) was introduced perpendicularly to the skin. After piercing the costo-transverse ligament, a proper muscular response of the intercostal muscles of the corresponding level was obtained and the needle’s tip was manipulated into a position allowing a muscular response while reducing the stimulating current to 0.4–0.6 mA, and the depth was then measured. The insulated needle was then removed and an 18 G Tuohy needle (Perifix, B. Braun Melsungen AG) was introduced at the same point and at the same depth previously measured. Even if inadvertent hitting of spinous process occurred (especially in obese patients), the distance was measured, and a Tuohy needle was advanced perpendicularly in the same track to hit the spinous process at the same depth previously measured, then withdrawn and redirected more caudad until it slips off of the transverse process, it was then advanced anteriorly and laterally approximately 1–1.5 cm. Loss of resistance by saline was done to confirm the space and to make catheter introduction easier. After negative aspiration of blood, air or cerebrospinal fluid, a standard epidural catheter was introduced cephalad and placed 4–5 cm into the space, and then fixed to the skin and the patient was then returned to the supine position. A bolus study dose of 2% lidocaine with adrenaline (1:200000) 5 ml was injected with monitoring of the conscious level, heart rate and blood pressure, to exclude spinal or epidural injection or pneumothorax; and the remaining 15 ml was injected after 5 min with aspiration, with injecting 5 ml every 5 min. Sensory loss was assessed by the loss of pin-prick sensation at the dermatomal distribution of the root being blocked first, then the sensory extent of the block was assessed later above and below the level of catheter introduction. After assuring adequate anaesthesia, and the patient was vitally stable, without any manifestations of local anaesthetic toxicity, the patient was transferred to the operating room. Midazolam, 2–5 mg, was given to patients of the C group.
General anaesthesia was then started, by the anaesthetist without identification of the patients with TPVB, to both the study group and the control group with intravenous fentanyl 100 μg and propofol 2–3 mg kg−1, and intubation was facilitated by atracurium 5 mg kg−1. Anaesthesia was maintained with isoflurane 1–1.5% in oxygen and mechanical ventilation. Heart rate and mean arterial blood pressure (MAP) were maintained within ±20% of the preoperative baseline, and IV bolus doses of fentanyl approximately 1 μg/kg were given if the MAP or heart rate increased more than 20% of the baseline.
After extubation following reversal of the relaxant effect and stopping isoflurane, the patients were shifted to the postanaesthesia care unit PACU, and a resident unaware of the anaesthetic technique collected postoperative patient data. The anaesthesia time was recorded, starting from monitor placement before the block till extubation in the study group, and the time of surgery started from intraoperative monitor placement until extubation.
The time at which the patients asked for the first rescue for analgesia was recorded, and a VAS pain score was recorded at 30 min, 1 h, 2 h, 4 h, 12 h, and 24 h. Analgesia for the control group was provided with IV increments of 2–3 mg morphine every 15 min until pain VAS score was <3, and a 5 ml bolus dose of lidocaine 2% and an infusion of 1% lidocaine 5 ml/h started for the patients in the PVB group. A 2–3 mg morphine bolus was given if pain persisted for 15 min after the bolus dose of lidocaine. The number of rescue doses of analgesia during the 24 h study period was recorded, and the VAS pain score was recorded at 30 min, 1 h, 2 h, 4 h, 12 h, and 24 h both at rest and with shoulder movements. Total intraoperative fentanyl dose administered and the total postoperative morphine consumption for each patient were recorded.
Side effects, such as nausea and vomiting, hypotension, respiratory complications as respiratory depression or pneumothorax, or local anaesthetic toxicity were recorded.
2.1 Statistical analysis
Quantitative data were expressed as mean ± standard deviation (SD). Unpaired Student’s t-tests or two-way analysis of variance (ANOVA) test was used for variable differences in groups, and Bonferroni correction tests were used for correction of multiple comparisons. Categorical data were analysed using χ2-test analysis or the Fisher Exact test, as appropriate. Statistical significance was defined as P < 0.05.
4 Results
All patients completed the study, there were no statistical differences between the two groups regarding the age, weight, and ASA physical status. No statistical differences in the time of surgery, but the time of anaesthesia was increased significantly in the PVB group than the control group due to adding the time of block .
Table 1 Patients’ characteristics and time of surgery and anaesthesia (min), values are expressed as mean ± SD.
There were highly significant decreases (p < 0.01) in both intraoperative fentanyl consumption and postoperative morphine consumption in the PVB group than the control group. There was also significant decrease in the number of patients who requested for analgesia and in the number of the boluses of morphine given on patient’s request .
Table 2 Intraoperative narcotic consumption, the first time to request for analgesia, and the number of times for requesting analgesia, values are expressed as mean ± SD.
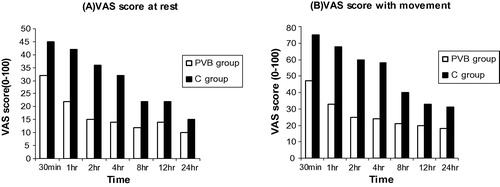
At rest, there were statistically significant decreases in the VAS in the PVB group than the C group (P < 0.05) at 30 min, 1 h, 2 h, 4 h, and 8 h. With shoulder movement, VAS was significantly lower in the PVB group than the C group at all times recorded. The decrease was highly significant (p < 0.01) at 1 h, 2 h, and 4 h at rest and with shoulder movement. In three patients in the PVB group, the loss of pin-brick sensation was not evident with increases of the intraoperative fentanyl and postoperative morphine consumption (failure rate 15%) ().
There were no major adverse events in either group requiring intervention in the operating room or in the PACU. No symptoms or signs of local anaesthetic toxicity, or pneumothorax were recorded in any patient after TPVB. The incidence of nausea and vomiting was lower in the TPVB (2/20) than the control group (4/20) but not statistically significant.
5 Discussion
The results of this study showed that TPVB with catheter application provides improvement in intraoperative and postoperative analgesia, less need for intraoperative and postoperative opioid analgesics, and without increases in side effects for patients who were candidates for major breast cancer surgery when compared to control group of patients under general anaesthesia.
TPVB is an old technique which was described by Hugo Sellheim in 1905, who used the technique to produce abdominal analgesia. TPVB was neglected until 1979, when Eason and Wyatt described a catheter technique [Citation5].
The thoracic paravertebral space (TPVS) is a wedge-shaped space that lies on either side of the vertebral column. The parietal pleura forms the anterolateral boundary, while the base is formed by the posterolateral aspect of the vertebral body, the intervertebral disc, the intervertebral foramen and its contents. The superior costotransverse ligament, which extends from the lower border of the transverse process above to the upper border of the transverse process below, forms the posterior wall of the TPVS. The apex of the space is continuous, with the intercostal space lateral to the tips of the transverse processes [Citation2,Citation4].
A thoracic paravertebral injection may remain localized to the level injected, or it may spread to the contiguous levels above and below, the intercostal space laterally, the epidural space medially, or a combination of the above to affect ipsilateral somatic and sympathetic nerves. It was evidenced that a mean distribution of the somatic block of five dermatomes, by loss of pinprick sensation, the mean distribution of the sympathetic block was eight epsilateral dermatomes by thermographic imaging [Citation2,Citation4–Citation6].
TPVB is technically easy to learn and perform, could be done in awake sedated patient. The failure rate in the study was 15%, which is high probably due to the small number of patients and due to catheter application.
The failure rate varies from 6.1% to 10% in other studies, which was comparable with that of other used regional anaesthetic techniques and reflects the technical difficulty in identifying the TPVS [Citation4,Citation7,Citation8].
Many approaches have been described. The classical technique, which was most commonly used, involves eliciting loss of resistance by air or saline as the superior costotransverse ligament is traversed. Using loss of resistance technique is subjective and may be misleading. In this study, TPVB was made easier and safer by knowing the depth of the space before needle insertion by the use of nerve stimulator. It could be used in awake sedated patients, but in some obese patients the response may not appear and the patient must be asked about sensation of muscle contraction [Citation2,Citation4].
Lang and Scott in 2002 and Boezaart et al. in 2006 reported that the use of nerve stimulator is simple and allows precise correlations to be made between anatomy, physiology (motor responses, electrically elicited paraesthesiae, and reproduction of pain in the targeted dermatome/s) and clinical effect. Also it does offer some additional degree of safety [Citation9,Citation10].
Lidocaine with adrenaline was used in this study to increase safety of using large doses and to avoid the marcain’s cardiotoxicity, especially with the presence of catheter. Lidocaine was proved to be an attractive alternative to marcaine in TPVB in children for its shorter elimination half-life and lower cardiotoxicity [Citation11].
TPVB by injection of local anaesthetic at a single level at T4 or at multiple levels in conjunction with heavy sedation or with general anaesthesia was evaluated for a long time for anaesthesia in breast surgery. It was found to be effective and safe for anaesthesia and postoperative analgesia, with a high degree of patient satisfaction and minimal complications. TPVB resulted in better postoperative pain control and earlier resumption of diet compared with general anaesthesia. Single or multiple injections techniques are limited by the duration of the local anaesthetic; and multilevel injections have definitely another disadvantage for a technique that requires positioning and multiple injections [Citation3,Citation12–Citation18].
The use of paravertebral catheter to provide long-lasting analgesia has been described in adults and children, for breast surgery, and for other surgeries [Citation19,Citation20]. In this study, catheter application was very effective in postoperative analgesia and in reduction of the analgesics used. It also provides safety in local anaesthetic administration as multiple small bolus doses. The potential problems with TPVB with catheter application are intrapleural catheter placement and subarachnoid or epidural injection. These complications were not recorded, due to the adherence to the anatomical study, the use of nerve stimulator, multiple aspirations and injections, with good patient monitoring.
Recently, fentanyl or clonidine was added to diluted levobupivacaine with intra- and postoperative paravertebral analgesia in patients undergoing breast surgery under general anaesthesia. Other studies were done to assess the efficacy and ability to use a patient-controlled paravertebral analgesia for breast cancer surgery, and satisfactory analgesia was provided [Citation21,Citation22].
The paravertebral block has been used successfully for analgesia in surgeries other than breast surgery as abdominal operations, hepatic surgery and thoracic surgery. TPVB was proved to be effective for analgesia for fracture ribs, penetrating trauma and for patients with post-herpetic neuralgia refractory to medical therapy. [Citation4,Citation23–Citation26].
Naja et al [Citation8] looked at complications of PVB following thoracic and lumbar paravertebral blocks performed in 620 adults and 42 children. The complications recorded were: inadvertent vascular puncture (6.8%); hypotension (4.0%); haematoma (2.4%); pain at site of skin puncture (1.3%); signs of epidural or intrathecal spread (1.0%); pleural puncture (0.8%); pneumothorax (0.5%).
Few side effects appear after TPVB, nearly similar to those in the patients of the control group. We found no evidence of local anaesthetic toxicity in any patients of the study group. Also, hypotension was not recorded with the block for any of the patients in the study. Hypotension was reported to be not common in other studies after TPVB in normovolemic patients because of unilateral sympathetic blockade [Citation2,Citation4,Citation8].
The technique of paravertebral block sometimes does not gain acceptance because of requirement of more preoperative time for catheter insertion and block. This was clear in this study by the significant increase in the time of anaesthesia in the study group than the control group, but this problem could be solved by the early starting of the block before the operation starting time in the monitored holding area.
Because the paravertebral space lies adjacent to the intervertebral foramina, inadvertent spread into the epidural space may occur, the first small bolus dose with monitoring of anaesthesia to the other side and monitoring of pulse rate and blood pressure is very essential. Further, aspiration before injection is also mandatory because the space contains vascular structures.
The most serious complication of the technique of thoracic PVB is the potential occurrence of pneumothorax by deep needle penetration. If pneumothorax is not a possibility, as after thoracotomy in which chest drains are placed for all cases, lung disease may be a strong indication for thoracic paravertebral block to promote breathing [Citation2,Citation4,Citation8]
This complication was not present in the study by the beneficial use of the nerve stimulator to evaluate the depth of the block without inadvertent deep penetration and pleural injury.
6 Conclusion
Thoracic paravertebral block is a simple, easy-to-learn technique with few contraindications and is associated with a low incidence of complications. Continuous TPVB provides effective pain relief, significant opioid sparing, and also causes less painful restricted movement of the shoulder, with very few side effects after breast cancer surgery. Thoracic paravertebral somatic nerve block may be an alternative to general anaesthesia for major unilateral breast surgery with heavy sedation or to be combined with light general anaesthesia.
Notes
Available online 20 May 2011
References
- H.VilaJ.LiuD.KavasmaneckParavertebral block new benefits from an old procedureCurr Opin Anaesthesiol2042007316318
- M.KarmakarK.ManojThoracic paravertebral blockAnesthesiology9532001771780
- S.KleinA.BerghS.SteeleThoracic paravertebral block for breast surgeryAnesth Analg90200014021405
- A.NaidooThoracic paravertebral blockAnaesthetics UKZN2008128
- M.J.EasonR.WyattParavertebral thoracic block-a reappraisalAnaesthesia341979638642
- S.CheemaD.IlsleyJ.RichardsonA thermographic study of paravertebral analgesiaAnaesthesia5021995118121
- E.CoveneyC.WeltzR.GreengrassUse of paravertebral block anesthesia in the surgical management of breast cancer Experience in 156 cases.Ann Surg2271998496501
- Z.NajaP.LönnqvistSomatic paravertebral nerve blockade. Incidence of failed block and complicationsAnaesthesia5612200111841188
- A.LangA.ScottThe use of a nerve stimulator for thoracic paravertebral blockAnesthesiology9722002521
- A.BoezaartM.RobertContinuous thoracic paravertebral block for major breast surgeryAm Soc Reg Anesth Pain Med2006470476
- P.A.LönnqvistPlasma concentrations of lignocaine after thoracic paravertebral blockade in infants and childrenAnaesthesia481993958960
- R.GreengrassF.O’BrienK.LyerlyParavertebral block for breast cancer surgeryCan J Anaesth431996858861
- M.NajarianJ.JohnsonJ.LandercasperParavertebral block: an alternative to general anesthesia in breast cancer surgeryAm Surg69320031318
- P.KairaluomaM.BachmannA.KorpinenSingle-injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsyAnesth Analg99200418371843
- R.CooterG.RudkinS.GardinerDay case breast augmentation under paravertebral blockade: a prospective study of 100 consecutive patientsAesth Plast Surg3162007666673
- A.DabbaghH.ElyasiThe role of paravertebral block in decreasing postoperative pain in elective breast surgeriesMed Sci Monit13102007464467
- J.MollerL.NikolajsenS.RodtThoracic paravertebral block for breast cancer surgery: A randomized double-blind studyAnesth Analg105200718481851
- C.BuckenmaierS.SteeleK.NielsenBilateral continuous paravertebral catheters for reduction mammoplastyActa Anaesthesiol Scand468200210421045
- C.BuckenmaierS.KleinK.NielsenContinuous paravertebral catheter and outpatient infusion for breast surgeryAnesth Analg972003715717
- Burlacu C, Frizelle H, Moriarty D, et al. Fentanyl and clonidine as adjunctive analgesics with levobupivacaine in paravertebral analgesia for breast surgery 2006;61(10):932–7.
- R.McElwainN.FreirC.BurlacuThe feasibility of patient-controlled paravertebral analgesia for major breast cancer surgery: A prospective, randomized, double-blind comparison of two regimensAnesth Analg1072008665668
- W.CulpC.TimothyM.WrightParavertebral block: an improved method of pain control in percutaneous transhepatic biliary drainageCardiovasc Intervent Radiol296200610151021
- M.KarmakarM.CheungG.LamRight thoracic paravertebral analgesia for hepatectomy (case report)Br J Anaesth9332004458461
- J.RichardsonS.SabanathanJ.JonesA prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responsesBr J Anaesth8331999387392
- Z.NajaA.RafM.El RajabNerve stimulator-guided paravertebral blockade combined with sevoflurane sedation versus general anesthesia with systemic analgesia for postherniorrhaphy pain relief in children: A prospective randomized trialPain Reg Anesth10332005600605
- Z.NajaH.MaalikiM.Al-TannirRepetitive paravertebral nerve block using a catheter technique for pain relief in post-herpetic neuralgiaBr J Anaesth9632006381383