Abstract
Background
Although being used off-label, the utility of dexmedetomidine in pediatric settings is increasing. Alpha-2 agonists have peripheral analgesic effects. This prospective, randomized, double-blind, Placebo-controlled study was designed to evaluate the safety and efficacy of dexmedetomidine single intraoperative preincisional dose in pediatric patients undergoing tonsillectomy and adenoidectomy.
Patients and methods
Eighty-four children (5–12 years) were randomized into three groups: DEX.IV (n = 28) received dexmedetomidine 1 μg/kg iv. infusion in 10 min, DEX.PT (n = 28) received dexmedetomidine 1 μg/kg peritonsillar infiltration, and the Placebo controls (n = 28). Assessment parameters included pain, sedation, hemodynamics, and adverse effects.
Results
Intraoperative dexmedetomidine administration resulted in a significant reduction in pain scores postoperatively in the DEX.IV and DEX.PT groups, with no significant difference between them. The time to first postoperative analgesic request was significantly prolonged in DEX.IV (583.45 ± 157.94 min, P < 0.000) and DEX.PT (537.61 ± 106.17 min, P < 0.000) groups compared with the Placebo group (119.75 ± 43.44 min). Similarly, a significantly lower paracetamol consumption during the first postoperative day was recorded in the DEX.IV (459.37 ± 114.82 mg, P < 0.000) and DEX.PT (475.38 ± 143.11 mg, P < 0.000) groups, than in the Placebo group (705.00 ± 249.27 mg), with no significant difference between DEX.IV and DEX.PT groups. Patients in the DEX.IV group exhibited significantly prolonged extubation times ((13.83 ± 3.38 min, P < 0.000) and significantly higher mean Ramsay sedation scores at 15, 30, 60, 120, and 180 min postoperative (P < 0.000), compared with DEX.PT and Placebo groups. The mean intraoperative heart rates were significantly slower in DEX.IV group during and after the intravenous infusion of dexmedetomidine and at 15th min intraoperative (p < 0.05), compared with DEX.PT and Placebo groups, with no significant differences in mean heart rates among the groups in other time points measured. Patients in DEX.PT group had a significantly higher total oral intake in first day postoperative (P < 0.000) and a significantly higher family satisfaction (p < 0.000), compared with DEX.IV and Placebo groups.
Conclusion
Peritonsillar infiltration or iv. dexmedetomidine similarly enhanced the postoperative analgesia after tonsillectomy in pediatric patients. However, locally applied dexmedetomidine was associated with no systemic effects, higher total oral intake in first day postoperative, and higher family satisfaction.
1 Introduction
Dexmedetomidine (DEX) (Precedex; Hospira, Inc.Lake Forest, IL) is a highly selective α2-adrenoceptor agonist recently introduced to anesthesia practice producing dose-dependent sedation, anxiolysis, and analgesia (involving spinal and supraspinal sites), without respiratory depression [Citation1,Citation2]. Compared with Clonidine, DEX is a more specific and selective α2-adrenergic agonist with a shorter elimination half-life [Citation3]. Its only approved indication by US FDA (1999) is the provision of short term sedation (<24 h) in adult patients in ICU settings who are initially intubated and mechanically ventilated [Citation4].
DEX is being used off-label as an adjunctive agent in pediatric patients for sedation and analgesia; in critical care unit, during non-invasive (e.g., Magnetic resonance imaging) and invasive procedures (e.g., cardiac catheterization and endoscopy) [Citation5]. It may also decrease opioid usage and anesthesia requirements as seen from adult data [Citation6], prevent emergence delirium [Citation7] and postanesthesia shivering [Citation8].
Pediatric experiences in the literature are in the form of small studies and case reports [Citation1], with limited data regarding the use of dexmedetomidine as a premedication for anxiolysis and postoperative pain. The appropriate dose and route of administration of such application are still under investigation.
Tonsillectomy and adenoidectomy are among the most common surgical procedures in pediatric population, and posttonsillectomy pain is still a debate [Citation9,Citation10], affecting the analgesic consumption, hospital stay, oral intake, and return to regular activity [Citation10,Citation11].
Alpha-2 adrenergic agonists have peripheral analgesic effects [Citation12]. Therefore, this study was designed to compare the effects of dexmedetomidine 1 μg/kg single intraoperative dose given by two different routes of administration, intravenous and peritonsillar infiltration, on postoperative recovery including pain, sedation, and hemodynamics in pediatric patients undergoing tonsillectomy and adenoidectomy, and the recording of any adverse effects that might develop during the 24-h study period.
2 Patients and methods
This prospective, randomized, double-blind, Placebo-controlled study was approved from the local research Ethics Committee in the faculty of medicine, Assiut University, Egypt. The study included 84 patients (aged 5–12 years), ASA I-II scheduled for elective tonsillectomy with or without adenoidectomy (using the surgical retraction and bipolar diathermy if indicated). An informed written consent was obtained from all the patient's legal guardians.
The indications for tonsillectomy were recurrent or chronic tonsillitis. Excluded from the study are patients with the following conditions: obstructive sleep apnea syndrome (whether confirmed by a polysomnography test or not), previous peritonsillar abscess formation, cardiovascular, liver or renal disease, unsatisfactory preoperative peripheral arterial oxygen saturation, neurological or psychiatric disease, coagulation disturbances, relevant drug allergies, difficulties in pain perception and assessment, and lastly, children with a BMI >95th percentile for age .
Based on a computer-generated randomization method, patients were enrolled into three groups: I-DEX.IV: patients (n = 28) received dexmedetomidine 1 μg/kg diluted in 50 ml saline 0.9% and given by iv. infusion in 10 min after induction of anesthesia and peritonsillar saline infiltration (2 ml per tonsil). II-DEX.PT: patients (n = 28) received dexmedetomidine 1 μg/kg diluted in 4 ml saline 0.9% and given by peritonsillar infiltration (2 ml per tonsil), after intubation 3–5 min before start of surgery. Using a 25-gauge spinal needle connected to a syringe, the tonsillar bed and peritonsillar tissues on both sides were infiltrated in a fan-wise injections from the superior and inferior poles of the fossa. Patients also received 50 ml saline 0.9% infusion after anesthetic induction in 10 min. III-Placebo: patients (n = 28) received 50 ml saline 0.9% iv. infusion and 4 ml saline 0.9% peritonsillar infiltration (2 ml per tonsil), at the same time points mentioned above. The dose of dexmedetomidine selected (1 μg/kg) was based on previous studies that confirmed the analgesic efficacy of dexmedetomidine 1 μg/kg rather than 0.5 μg/kg and 0.75 μg/kg [Citation13].
Preoperatively, patients (7–12 years) and their guardians were instructed in how to use the pain assessment tool of verbal numeric rating pain scale [Citation14] ranging from 0 to 10 (with zero = no pain and 10 = the worst pain imaginable).
The anesthetic regimen was standardized; it included induction with propofol 2–3 mg/kg and atracurium besylate 0.5 mg/kg to facilitate endotracheal intubation and maintenance with isoflurane and oxygen/air mixture. Intraoperative monitoring included ECG, pulse oximeter, non-invasive blood pressure, and end-tidal CO2. An intravenous antibiotic and dexamethazone (0.2 mg/kg, max. dose 8 mg) were administered. No opioids, NSAIDS, paracetamol, or additional propofol were used during the procedure. At the end of the operation, neuromuscular blockade was antagonized with neostigmine 0.05 mg/kg and atropine 0.02 mg/kg, and patients were turned aside in the posttonsillectomy recovery position. Patients extubated awake after confirming the return of airway protective reflexes and transported to (PACU), with supplemental oxygen that discontinued if the child could sustain a SaO2% >95% for 5 min on room air. After attaining an Aldrete score [Citation15] ⩾9, patients were moved from PACU to the ward. Patient care or data collection personnel and the surgeon were blinded to the patient assignment.
Operative room data were included; heart rate and mean arterial blood pressure were continuously monitored and recorded preoperatively, before, during, and after the administration of the study solutions, and at 15th, 20th, 25th, and 30th min intraoperative; anesthesia time (from induction of anesthesia till extubation); operative time (from start of surgery till end of bleeding control); and extubation time (from discontinuation of anesthesia till extubation).
PACU and ward data were included; heart rate and mean arterial blood pressure were measured and recorded on arrival in the PACU (0 min) and at 15th, 30th, 45th, and 60th min postoperative; Ramsay sedation scale [Citation16] scores measured at 0 (on arrival in the PACU), 15, 30, 60, 120, 180 and 240 min postoperative; time to first and subsequent request of supplemental analgesics and total dose of rescue analgesics consumed postoperative; total oral intake in first 24 h postoperative including fluids and semisolids. The Children's Hospital of Eastern Ontario (CHEOPS) [Citation17] pain scale (children <7 years) or the verbal numeric rating scale [Citation14] (children 7–12 years) were measured at 0 (on arrival at PACU), 30, 60, and 90 min postoperative. They were also recorded at 2, 6, and 12 h postoperative, and the mean value of the three values was calculated and recorded as the mean CHEOPS or VNRS scores in the first day postoperative. Paracetamol (Perfalgan®, Bristol Meyers Squibb, New York) 15 mg/kg iv. was given if requested and if VNRS scores were ⩾3 or CHEOPES scores were >8.
Perioperative side effects were treated and recorded (e.g., hypertension, hypotension, bradycardia, tachycardia, arrhythmia, hypoxia, nausea, vomiting, excess secretions, bleeding and respiratory depression). The children's families graded their satisfaction regarding analgesia (very satisfied, mildly satisfied, or not satisfied) at the end of the 24-h study period.
3 Statistical analysis
The primary outcome measure was the total dose of analgesics consumed in the first day postoperative. Secondary outcome measures were time to first request of rescue analgesics, number of patients who needed more than 1 analgesic dose, time to extubation, Ramsay sedation score, heart rate, mean arterial pressure, total oral intake in first 24 h postoperative, and the incidence of postoperative side effects.
Sample size: in our institution, we annually anesthetize 480–768 (10–16/week) tonsillectomy and adenoidectomy case suffering from chronic or recurrent tonsillitis. Previous studies on adults reported 33% decrease in morphine use postoperatively when using dexmedetomidine iv. 0.4 μg/kg [Citation18] and 66% decrease when using dexmedetomidine 1 μ/kg iv. [Citation19], with no available published data about dexmedetomidine peritonsillar administration. Our power analysis was based on estimating a 20% reduction in analgesic requirements in a sample population of 600. A calculated sample size of 28 would have an 80% power of detecting a difference at 0.05 level of significance, using a confidence interval of 95%.
Analysis was performed using SPSS version 17 (Chicago-USA). Data were presented as mean ± SD, numbers, frequencies, and percentages. ANOVA followed by post hoc test were used for comparison of parametric data. Kruskal–Wallis test was used to compare non-parametric data while Mann–Whitney used to compare between two groups. Chi-square test was used for comparison between percentages and frequencies. P < 0.05 was considered significant.
4 Results
One hundred fifty-six patients who qualified for the study were approached: 84 consented and 72 refused. The consented 84 patients were equally distributed into the three groups, with no patient dropouts. All procedures were performed by 1 of 4 otolaryngologic surgeons with an even distribution of cases among the 4. There were no differences among the groups of patients with regard to age, weight, gender, and duration of surgery (). The time to extubation was significantly prolonged in the DEX.IV group (13.83 ± 3.38 min, P < 0.000), compared with the DEX.PT (6.19 ± 1.80 min) and Placebo (6.57 ± 1.77 min) groups, leading to a significantly prolonged anesthesia times in DEX.IV. (53.67 ± 7.29 min, P < 0.000), but not the DEX.PT (46.75 ± 6.88 min) or the Placebo (46.67 ± 6.11 min) groups, respectively ().The highest mean values of 24-h total oral intake for fluids and semisolids were achieved in the DEX.PT group (687.14 ± 123.10 ml and 607.85 ± 143.04 ml, P < 0.000), compared to the DEX.IV (648.57 ± 88.89 ml and 473.21 ± 146.51 ml) and Placebo (592.85 ± 132.10 ml and 332.14 ± 108.84 ml) groups, respectively ().
Table 1 Demographic and recovery characteristics.
The mean time to first request of rescue analgesia was significantly prolonged in DEX.IV (583.45 ± 157.94 min, P < 0.000) and DEX.PT (537.61 ± 106.17 min, P < 0.000) groups compared to the Placebo group (119.75 ± 43.44 min). The number of patients required >1 rescue analgesic dose was higher in the Placebo group (n = 11/30.8%), compared to DEX.IV (n = 2/5.6%) and DEX.PT (n = 2/5.6%) groups. The mean total dose of iv. paracetamol rescue analgesia consumed in first 24 h postoperative was significantly lower in DEX.IV (459.37 ± 114.82 mg, P < 0.000), and DEX.PT (475.38 ± 143.11 mg, P < 0.000) groups, but not the Placebo group (705.00 ± 249.27 mg) (). Pain scores recorded at 0, 30, 60, and 90 min postoperative were significantly lower in DEX.IV and DEX.PT groups compared to Placebo (). Moreover, the mean CHEOPS and VNRS pain scores in the first day postoperative were significantly reduced in DEX.IV (6.98 ± 0.86, p < 0.001 and 2.45 ± 0.31, p < 0.000) and DEX.PT (7.12 ± 0.81, p < 0.003 and 2.59 ± 0.27, p < 0.001) groups, compared to the Placebo group (9.34 ± 0.91 and 3.88 ± 0.34), respectively.
Table 2 Pain profile.
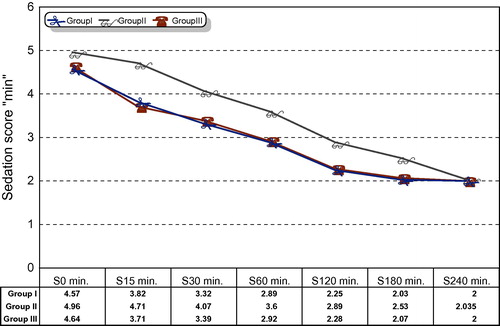
The Ramsay sedation score in the first 240 min postoperative decreased over time in all three groups. However, mean sedation scores were significantly higher in DEX.IV group (P < 0.000) compared to DEX.PT and Placebo groups at 15, 30, 60, 120, and 180 min postoperative (, ).
Table 3 Ramsay sedation score.
The mean intraoperative heart rates were significantly slower in DEX.IV group during and after the intravenous infusion of dexmedetomidine and at 15th min intraoperative (p < 0.05), compared with DEX.PT and Placebo groups (). Moreover, two patients in the DEX.IV group manifested significant intraoperative bradycardia (>20% of baseline). The first patient showed bradycardia and desaturation (SaO2% = 91%) lasted < 60 s during the infusion of dexmedetomidine and resolved without treatment. The second patient had two attacks of bradycardia, after the end of dexmedetomidine iv. infusion and at the end of operation that necessitated iv. atropine administration (20 μg/kg). Otherwise, there were no significant differences in mean heart rates among the groups in other time points measured ().
Table 4 The heart rate (beat/min).
Of the 84 patients, 8 had emesis, 4 of them vomited once, and 6 complained from excessive secretions with no intergroup statistical differences. No patient reported prolonged supplemental oxygen requirements, hypo or hypertension, tachycardia, arrhythmia, respiratory depression, or tonsillar bed bleeding. Finally, a significantly higher family satisfaction () was recorded in DEX.PT group (p < 0.000), compared to DEX.IV and Placebo groups.
Table 5 Family satisfaction index.
5 Discussion
The main finding in this study was that both intravenous and peritonsillar dexmedetomidine in a dose of 1 μg/kg administered intraoperatively before the start of adenotonsillectomy surgery, enhanced postoperative pain relief, prolonged time to first request and reduced the need for postoperative analgesia. Peritonsillar dexmedetomidine produced comparable analgesia, earlier recovery, less sedation, less bradycardia, higher total oral intake in first day postoperative, and a higher family satisfaction compared to intravenous dexmedetomidine.
The analgesic effects of α2-adrenergic agonists could be mediated through supraspinal, spinal and peripheral actions [Citation20]. The reduction in analgesic requirements in this study was in accordance with previous adult [Citation19,Citation21] and pediatric [Citation22–Citation24] studies which concluded that intraoperative administration of dexmedetomidine significantly reduces postoperative opioid analgesic requirements. The difference in our study is the use of paracetamol iv. rescue analgesia, as our institution protocols prefer non-opioid analgesia for posttonsillectomy pain.
In this study, the lack of systemic effects in the peritonsillar dexmedetomidine group suggests the possibility of a direct local action. But we cannot exclude a central analgesic effect resulting from systemic absorption; because of the similar analgesic profile observed between intravenous and peritonsillar groups, and the rich vascularity of the peritonsillar area. Unfortunately, we did not measure the plasma concentration of dexmedetomidine to correlate it with the clinical findings that might have confirmed the local effects. Further studies are needed to define the optimum analgesic dose of peritonsillar dexmedetomidine and to clarify its local adverse effects in pediatric population. A future research question arises: can we select between combined effects of dexmedetomidine by changing the route of administration?
The sedative effect of dexmedetomidine is characterized by being short term and easily arousable “Arousable sedation” [Citation25]. Other clinically available sedatives failed to produce such sedation. This feature was shown by Hall and colleagues who used the Bispectral index system and psychometric tests such as the Visual Analogue Scale for Sedation, Observer's Assessment of Alertness/Sedation Scale, Digit Symbol Substitution Scale, and specific memory tests. In accordance with our results, these parameters showed reduced values by dexmedetomidine administration that returned to baseline 4 h after treatment [Citation26]. Moreover, the Bispectral index system returned from 60 to 65 before stimulus back to normal values when encouraged [Citation26]. A larger European phase III trial underlined these findings, stating that even complex tasks, such as communication by pen and paper, are possible under dexmedetomidine primary therapy [Citation25].
In our study, the analgesic effect of iv. dexmedetomidine 1 μg/kg was appreciated; however, its prolonged recovery times and arousable short term sedation annoyed the children's next of kin and delayed oral intake. This study was investigated in healthy subjects undergoing a relatively moderate operation under ambulatory conditions. And so, even mild sedation for 2–3 h postoperative delays the time to discharge readiness and time of first oral intake. Such sedation would be preferable in patients undergoing major operations such as cardiothoracic surgery and in critical care settings. For dexmedetomidine, appropriate patient selection is crucial [Citation5], and also the type of surgical procedure is important. Procedure-specific acute pain management guidelines may be helpful, taking into consideration that the risk–benefit ratio of different analgesics may vary according to the surgical procedure [Citation27–Citation29].
In the current study, slower intraoperative mean heart rates were observed during and after the intravenous infusion of dexmedetomidine 1 μg/kg (including two cases with significant bradycardia). One of the side effects with the use of dexmedetomidine is severe bradycardia [Citation30–Citation32]. The administration of a systemic bolus of 1 μg/kg dexmedetomidine initially results in a transient increase in the blood pressure and reflex decrease in heart rate, especially in younger, healthy patients [Citation33]. It can be explained, firstly, by peripheral α2B-adrenoceptor stimulation of vascular smooth muscle and can be attenuated by a slow infusion over 10 min or more [Citation26]. And secondly, due to the stimulation of presynaptic α2-adrenoceptors and decreased norepinephrine release [Citation34]. In accordance with our results, these effects were temporary and could be managed successfully with atropine or ephedrine and volume infusions [Citation35]. Appropriate patient selection is crucial; patients who are hypovolaemic, severely vasoconstricted, with fixed stroke volume, reduced myocardial function, depend on a high level of sympathetic tone should not receive dexmedetomidine.
Dexmedetomidine seems to have a few respiratory side effects [Citation36], and receptor binding studies suggest that its effect on respiration should be minor. Belleville et al. reported episodes of obstructive apnea in a group of patients who received high doses of the drug [Citation36]. These effects were seen more commonly with doses of 1 or 2 μg/kg given over 2 min, doses that provide deep sedation. The obstructive respiration pattern and irregular breathing seen with such doses are probably related more to deep sedation and anatomical features of the patients, implying a great caution when using dexmedetomidine in patients with obstructive sleep apnea syndrome. Moreover, the coadministration of dexmedetomidine with anesthetic agents, sedatives, hypnotics, or opioids is likely to cause additive effects [Citation34]. In our study, no patient exhibited signs of airway obstruction or prolonged oxygen requirement in PACU. To avoid conflicting results, we excluded from our study patients with obstructive sleep apnea whether confirmed by polysomnography or not. Future studies are needed to investigate the incidence of airway obstruction in children with obstructive sleep apnea syndrome receiving dexmedetomidine.
In conclusion, peritonsillar infiltration or iv. dexmedetomidine similarly enhanced postoperative analgesia after adenotonsillectomy in pediatric patients. However, the locally applied dexmedetomidine was associated with no systemic effects, higher total oral intake in first day postoperative, and higher family satisfaction. For pediatric administration of dexmedetomidine, appropriate patient and appropriate surgical procedure selection are crucial. Future studies are needed to define the optimum dosage requirements for different pediatric subpopulations.
Conflict of interest
There was no conflict of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- Z.P.KhanC.N.FergusonR.M.Jonesα2 and imidazoline receptor agonists: their pharmacology and therapeutic roleAnaesthesia541999146165
- M.MazeC.ScarfiniF.CavaliereNew agents for sedation in the intensive care unitCrit Care Clin172001881897
- A.BekkerM.K.SturaitisDexmedetomidine for neurological surgeryNeurosurgery5712005ONS-1ONS-8
- J.D.TobiasSedation and analgesia in paediatric intensive care units: a guide to drug selection and usePaediatr Drugs11999109126
- H.PhanM.C.NahataClinical uses of dexmedetomidine in pediatric patientsPediatr Drugs10120084969
- N.L.BhanaK.L.GoaK.J.McClellanDexmedetomidineDrugs592000263268
- G.GulerA.AkinZ.TosumSingle-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomyPediatr Anesth152005762766
- R.B.EasleyK.M.BradyJ.D.TobiasDexmedetomidine for the treatment of postanesthesia shivering in childrenPediatr Anesth172007341346
- F.I.CatlinW.J.GrimesThe effect of steroid therapy on recovery from tonsillectomy in childrenArch Otolaryngol Head Neck Surg11761991649652
- M.J.CortneyD.CabraalTramadol vs diclofenac for posttonsillectomy analgesiaArch Otolaryngol Head Neck Surg12742001385388
- A.G.TomaJ.BlanshardN.Eynon-LewisM.W.BridgerPosttonsillectomy pain: the first ten daysJ Laryngol Otol109101995963964
- R.R.Al-MetwalliH.A.MowafiS.A.IsmailA.K.SiddiquiA.M.Al-GhamdiM.A.ShafiEffect of intra-articular dexmedetomidine on postoperative analgesia after arthroscopic knee surgeryBr J Anaesth10132008395399
- O.A.OlutoyeC.D.GloverJ.W.DiefenderferM.McGilberryM.M.WyattD.R.LarrierThe effect of intraoperative dexmedetomidine on postoperative analgesia and sedation in pediatric patients undergoing tonsillectomy and adenoidectomyAnesth Analg1112010490495
- S.W.HennebergL.B.NilsonAcute pediatric painAnaesth Crital Care2007003009
- J.A.AldreteD.KroulikA postanesthetic recovery scoreAnesth Analg491970924934
- M.A.RamsayT.M.SavageB.R.SimpsonControlled sedation with alphaxalone–alphadoloneBMJ21974656659
- P.J.McGrathG.JohnsonJ.SchillingerCHEOPS: a behavioural scale for rating postoperative pain in childrenAdv Pain Res Ther91985395402
- M.S.AhoO.A.ErkolaH.ScheininA.M.LehtinenK.T.KorttilaEffect of intravenously administered dexmedetomidine on pain after laparoscopic tubal ligationAnesth Analg731991112118
- S.R.ArianR.M.RuehlowT.D.UhrichT.J.EbertThe efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgeryAnesth Analg982004153158
- T.J.EbertJ.E.HallJ.A.BarneyT.D.UlrichM.D.ColincoThe effects of increasing plasma concentrations of dexmedetomidine in humansAnesthesiology932000382394
- N.M.BulowN.V.BarbosaJ.B.RochaOpioid consumption in total intravenous anesthesia is reduced with dexmedetomidine: a comparative study with remifentanil in gynecologic videolaparoscopic surgeryJ Clin Anesth192007280285
- K.R.Al-ZabenI.Y.QudaisatS.M.Al-GhanemI.M.MassadM.M.Al-MustafaA.S.Al-OwedidiIntraoperative administration of dexmedetomidine reduces the analgesic requirements for children undergoing hypospadius surgeryEur J Anaesthesiol272010247252
- A.M.El-HennawyA.M.Abd-ElwahabA.M.Abd-ElmaksoudH.S.El-OzairyS.R.BoulisAddition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in childrenBr J Anaesth1032009268274
- I.SaadawyA.BokerM.A.ElshahawyA.AlmazrooaS.MelibrayA.A.AbdellatifEffect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatricsActa Anaesthesiol Scand532009251256
- R.M.VennC.J.BradshawR.SpencerD.BrealeyE.CaudwellC.NaughtonPreliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in intensive care unitAnaesthesia54199911361142
- J.E.HallT.D.UhrichJ.A.BarneyS.R.ArainT.J.EbertSedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusionsAnesth Analg902000699705
- R.RosenquistJ.RosenbergPostoperative pain guidelinesReg Anesth Pain Med2842003279288
- A.GrayH.KehletF.BonnetN.RawalPredicting postoperative analgesia outcomes NNT League tables or procedure-specific evidence?Br J Anaeth9462005710714
- E.NeugebauerR.WilkinsonH.KehletS.SchugPROSPECT: a practical method for formulating evidence-based expert recommendations for the management of postoperative painSurg Endosc217200710471053
- K.P.MasonS.ZgleszewskiR.E.FormanC.StarkJ.A.DiNardoAn exaggerated hypertensive response to glycopyrrolate therapy for bradycardia associated with high dose dexmedetomidineAnesth Analg1082009906908
- K.A.CandiottiS.D.BergeseP.M.BokeschM.A.FeldmanW.Wise-mandleA.Y.Bekkerfor the MAC Study GroupMonitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trialAnesth Analg11020104756
- A.T.GerlachC.V.MurphyDexmedetomidine-associated bradycardia progressing to pulseless electrical activity: case report and review of the literaturePharmacotherapy2920091492
- B.C.BloorD.S.WardJ.P.BellevilleM.MazeEffects of intravenous dexmedetomidine in humans. II. Hemodynamic changesAnesthesiology77199211341142
- R.AantaaJ.KantoM.ScheininA.KallioH.ScheininDexmedetomidine, an alpha 2-adrenoceptor agonist, reduces anesthetic requirements for patients undergoing minor gynecologic surgeryAnesthesiology731990230235
- J.JalonenM.HynynenA.KuitunenH.HeikkilaPerttilaM.SalmenperaDexmedetomidine as an anesthetic adjuvant in coronary artery bypass graftingAnesthesiology861997331345
- J.P.BellevilleD.S.WardB.C.BloorM.MazeEffects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rateAnesthesiology77199211251133