Abstract
Background
Lornoxicam is a fairly new short-half oxicam with an improved tolerability profile. Our objective was to investigate the safety and efficacy of intravenous and peritonsillar infiltration of 8 mg lornoxicam on pain relief in children undergoing tonsillectomy.
Methods
In a double-blinded, placebo-controlled trial, 60 children were randomized into three groups; intravenous group (n = 20), received lornoxicam 8 mg iv., infiltration group (n = 20) received lornoxicam 8 mg peritonsillar infiltration, and placebo controls (n = 20). The verbal rating pain scale, time to first postoperative analgesic request, total analgesic consumption during 1st 24 h postoperative, platelet aggregometry before, 15 min, 2 and 24 h after study drug administration, intraoperative blood loss, postoperative bleeding, and adverse effects were evaluated.
Results
The time to first postoperative analgesic request was significantly prolonged in intravenous (318.75 ± 67.37 min) and infiltration (214.50 ± 43.06 min) groups compared with placebo group (66.75 ± 26.95 min). A significantly lower mean postoperative VRS scores and significantly reduced 1st day postoperative diclofenac consumption were recorded in iv. group (44.73 ± 9.31 mg), compared with infiltration (69.80 ± 38.71 mg) and placebo (87.8 ± 24.40 mg) groups. An increased intraoperative blood volume losses and intraoperative bleeding complains were observed in infiltration group (34.25 ± 11.93 ml), rather than in iv. (28.85 ± 10.01 ml) and placebo (24.75 ± 8.70 ml) groups. The (%) of platelet aggregation with ADP, collagen, and arachidonic acid was significantly reduced 15 min and 2 h after study drug administration with highest decreases in iv. group compared with infiltration and placebo groups. No patients reported postoperative bleeding or GIT adverse effects in the study.
Conclusion
Intraoperative preincisional intravenous lornoxicam enhanced postoperative analgesia after tonsillectomy in children. In comparison, the analgesic efficacy of locally applied lornoxicam was inferior to intravenous administration and was associated with increased incidence of intraoperative bleeding.
1 Introduction
Postoperative pain is one of the most troublesome aspects after tonsillectomy. Despite the use of various types of analgesics, the recovery period can be quite painful. Opioid-based vs. NSAIDS-based analgesic regimens is still a focus of continuous debate. Non-selective NSAIDS are claimed to cause increased incidence of perioperative bleeding caused by platelet function inhibition and gastrointestinal toxicity [Citation1–Citation3]. Although the mechanism of analgesic action (i.e., inhibition of cyclooxygenase enzyme COX I and II, leading to decreased prostaglandin synthesis) is the same as all available NSAIDS, the analgesic efficacy relative to side effects may vary from agent to agent [Citation4]. When used short term at the lowest effective dose, NSAIDS may provide for analgesic benefit without significant toxicity [Citation5].
Lornoxicam (Chlorotenoxicam, Xefo®) is a potent non-selective NSAID of the oxicam class with analgesic, anti-inflammatory, and antipyretic properties [Citation6]. It is rapidly eliminated with a short plasma elimination half life of 3–5 h [Citation7]. This short plasma half life may in part be responsible for the lornoxicam's reduced incidence of adverse effects [Citation7]. We designed this prospective study to demonstrate the postoperative analgesic efficacy and adverse effects of a single intraoperative dose of lornoxicam 8 mg given before the start of surgery in tonsillectomy patients (8–18 years). Two routes of administration were used in comparison, intravenous vs. peritonsillar infiltration.
2 Patients and methods
This study was approved by the Local Research Ethics Committee in the Faculty of Medicine, Assiut University, Egypt. After obtaining an informed written parental consent, 60 ASA physical status I–II patients aged 8–18 years and scheduled for elective tonsillectomy due to recurrent or chronic tonsillitis were included in the study. Excluded from the study, patients with known hypersensitivity to medication drugs, coagulation disorders, thrombocytopenia, bronchial asthma, significant cardiac, renal, pulmonary or hepatic disease, peptic ulcer, previous peritonsillar abscess formation, active bleeding for any cause, and patients received any analgesic medications within 24 h preoperative or antiplatelet medication within the past 2 weeks.
Using an online research randomizer (http://www.randomizer.org), patients were randomly allocated to three groups of 20 patients each to receive lornoxicam 8 mg iv. (intravenous group), or peritonsillar infiltration (infiltration group), or saline (placebo group), after induction of anesthesia before the start of the surgery.
The fasted unpremedicated patients received a standardized anesthetic technique that included induction with propofol 2–3 mg/kg iv. and atracurium besylate 0.5 mg/kg iv. to facilitate endotracheal intubation. Anesthesia was maintained with isoflurane in oxygen/air mixture. Patients were mechanically ventilated in ventilation parameters that maintain an end-tidal CO2 ∼ 32–35 mm Hg. Monitoring included electrocardiography (ECG), non-invasive blood pressure, peripheral arterial oxygen saturation (SaO2%), and end-tidal carbon dioxide (EtCO2).
After induction of anesthesia before the start of the surgery, patients in the placebo group received 10 ml of normal saline iv. and 4 ml saline by peritonsillar infiltration (2 ml each side). The lornoxicam intravenous group patients received iv. 8 mg lornoxicam diluted to 10 ml plus 4 ml saline by peritonsillar infiltration. The lornoxicam infiltration group patients received 8 mg lornoxicam diluted to 4 ml and infiltrated pericapsularly through the tonsillar bed and peritonsillar tissues in a fanwise direction from the superior to inferior poles of the fossa (2 ml each side), using a 25-gauge spinal needle over a syringe, Plus 10 ml saline iv. The attending anesthesiologist, surgeon and data collection personal were blinded to patient group assignment and to the nature of the study medication. Saline 0.9% was used for diluting study drugs, while Lactated Ringer's solution was used for fluid maintenance and deficit replacement.
An intravenous antibiotic and dexamethasone 0.2 mg/kg were administered. At the end of the surgery, anesthesia was discontinued and neuromuscular relaxation was reversed using neostigmine 40 μg/kg and atropine 20 μg/kg slowly intravenous, and patients were turned aside in the recovery position. Extubation performed awake after the return of protective airway reflexes. Patients were transported to PACU, where they discharged to the ward after attaining an Aldrete and Kroulik [Citation8] score >9. Intraoperative blood loss was roughly estimated by visual estimation of the blood volume lost in suction bottles, swab counting, and by the surgeon's estimation of bleeding using the following scale for perioperative bleeding assessment (0 = no bleeding, 1 = bleeding as usual, 2 = bleeding more than usual, 3 = profuse, 4 = excessive, and lastly 5 = excessive and continuously). Postoperative bleeding was also assessed by the same scale.
Pain intensity was assessed postoperatively by using the Verbal Rating scale (VRS) (0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain, and lastly 4 = excruciating pain). VRS assessments were performed at rest in the following time points; at arrival to PACU, 30 min, 1, 2, 3, 4, 5, 6, 12, and 24 h postoperative. Diclofenac sodium 1 mg/kg im was given if requested and if VRS scores were ⩾2, and the total consumption of rescue analgesics in the first 24 h postoperatively was calculated.
Any adverse effects in the 1st 24 h postoperative were treated and recorded including nausea and vomiting, bleeding, and any abnormal gastrointestinal manifestations.
Samples were withdrawn from larger veins on the forearm and antecubital areas in an atraumatic fashion with a minimum of stasis and avoiding contamination with iv fluids. Platelet counts (×109/μL) and prothrombin time (s) and concentration (%) were assessed preoperatively in all patients. Ten patients from control group, 15 patients from intravenous group, and 15 patients from the infiltration group were randomly selected to perform platelet aggregation studies at the following timepoints: before, 15 min, 2, and 24 h after the study medication was administered. Blood (4.5 ml) was withdrawn on Vacutainer tubes containing 0.5 ml trisodium citrate 3.8% (9:1 ratio) which rapidly centrifuged at 900–1200 rpm for 20 min, and then platelet rich plasma (200–350 × 103/μL) was taken to estimate platelet aggregation testing.
Platelet aggregation to adenosine diphosphate (ADP) (10 μM), collagen (2 μg/ml), and arachidonic acid (AA) (50 μmol/l) was performed using the optical method for the measurement of platelet aggregation (light transmitter method). Platelet aggregation was measured by percent change in light transmission using the platelet aggregation profiler model PAP-4 (Bio/data corporation) [Citation9], . The reagents were provided by Chrono-log Company (Havertown, PA, USA). Values more than 50% were considered normal.
3 Statistical analysis
Analysis was performed using SPSS version 17 (Chicago – USA). Data were presented as mean ± SD, numbers, frequencies, and percentages. ANOVA followed by post-hoc test was used for the comparison of parametric data. Kruskal–Wallis test was used to compare non-parametric data, while Mann–Whitney used to compare between two groups. Chi-square test was used for comparison between percentages and frequencies. P < 0.05 was considered significant.
4 Results
Ninety six patients were screened for eligibility to participate in this study, and 60 patients were subsequently consented and enrolled (n = 20 per group), with no patient drop outs. All procedures were performed by 1 of 4 otolaryngologic surgeons with an even distribution of cases among the four. There were no significant differences among the three groups with respect to age, gender, weight, ASA class, preoperative prothrombin time and concentration, preoperative platelet count, operation time, and anesthesia time ().
Table 1 Demographic data.
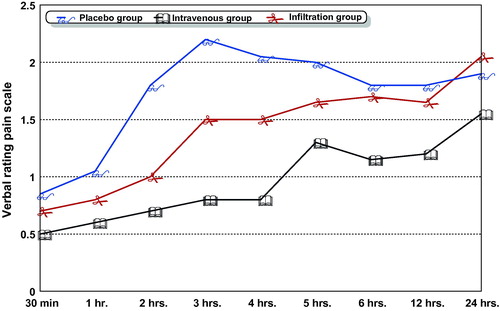
The time to first analgesic request was significantly prolonged in the intravenous group (318.75 ± 67.37 min), compared with the infiltration (214.50 ± 43.06 min, P < 0.000) and the placebo groups (66.75 ± 26.95 min, P < 0.000), respectively. The total diclofenac consumption in the first 24 h postoperative was significantly lower in intravenous group (44.73 ± 9.31 mg), compared with the placebo (87.8 ± 24.40 mg, P < 0.000) and infiltration (69.80 ± 38.71 mg, P < 0.04) groups, with a non-significant difference between the infiltration and placebo groups (). shows that the mean VRS pain scores recorded at rest in the intravenous group were significantly lower than the mean VRS pain scores in the placebo and infiltration groups at all recorded times. Mean VRS pain scores at the 24th hour postoperative were significantly higher in the infiltration group than that of other groups (P < 0.004).
Table 2 Postoperative pain profile.
The intraoperative blood volume loss was significantly higher in the infiltration group (34.25 ± 11.93 ml, P < 0.007), compared with placebo (24.75 ± 8.70 ml, P < 0.007) and intravenous (28.85 ± 10.01 ml) groups, with a non-significant difference between the intravenous and control groups. The surgeons’ estimation of intraoperative bleeding by intraoperative bleeding score showed that the number of patients exhibited intraoperative bleeding more than usual was significantly higher in the infiltration group (9.45%) compared with intravenous (4.20%, P < 0.000) and placebo (3.15%, P < 0.001) groups, respectively ().
Table 3 Bleeding assessments.
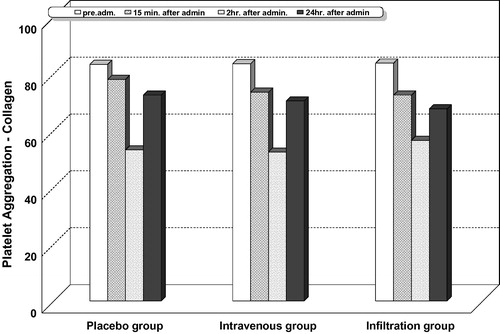
Intragroup comparison for platelet aggregation with adenosine diphosphate ( and ), collagen ( and ), and arachidonic acid ( and ) showed that platelet aggregation was significantly reduced at 15 min, 2, and 24 h after administration of study drugs in all the studied groups including the placebo. The highest percentage of decrease was observed at 2 h.
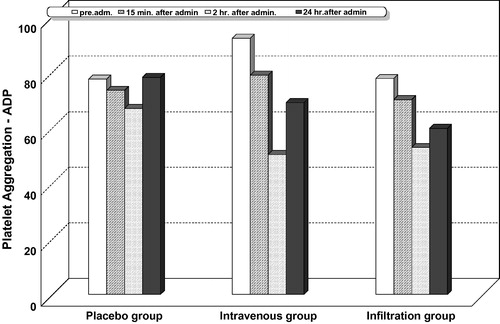
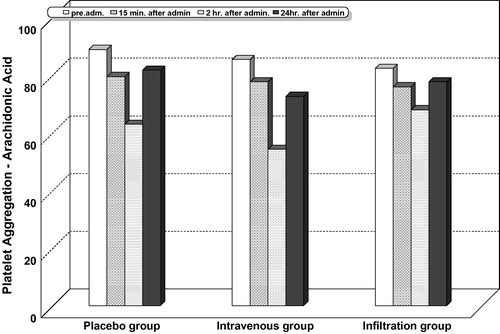
Table 4 Platlelet aggregation with adenosine diphosphate (ADP).
Table 5 Platlelet aggregation with collagen.
Table 6 Platlelet aggregation with arachidonic acid (AA).
Intergroup comparison for platelet aggregation with adenosine diphosphate ( and ), collagen ( and ), and arachidonic acid ( and ) showed that the highest percentage of reduction in platelet aggregation was significantly observed in the lornoxicam intravenous group at all the measured timepoints, compared with the infiltration and placebo groups. In this study, no patient recorded a platelet aggregation activity less than 50% with adenosine diphosphate, collagen, or arachidonic acid.
Of the 60 patients, 9 complained from nausea, 4 of them vomited once, 1 vomited twice and treated with iv. metclopramide, and 5 complained from excessive secretions with no intergroup statistical differences. No patients reported abnormal gastrointestinal manifestations, and no patient required reoperation for recurrent tonsillar bed bleeding.
5 Discussion
The current study had demonstrated that both intravenous and peritonsillar lornoxicam in a dose of 8 mg administered intraoperatively before the start of the adenotonsillectomy surgery, enhanced postoperative pain relief, prolonged time to first request, and reduced the need for postoperative analgesia. Although being better than placebo, the analgesic efficacy of locally applied lornoxicam was lagging behind that of iv. lornoxicam and was associated with increased intraoperative bleeding.
Of the panel of NSAIDS tested, lornoxicam was found to be the most potent balanced inhibitor of human cyclooxygenase (COX)-1/-2. The equipotent COX-isoenzyme inhibition is complemented by a marked inhibition of interleukin (IL)-6 and of inducible nitric oxide synthetase (iNOS) derived nitric oxide formation [Citation10]. These facts support the marked anti-inflammatory and analgesic activities of lornoxicam found in animal models [Citation11] as well as in clinical studies [Citation3,Citation10,Citation12].
Lornoxicam has been shown to be at least as effective as comparative NSAIDS and more effective than 10 mg morphine when used at doses ⩾8 mg to control pain after oral surgery [Citation12]. In addition, oral doses of lornoxicam of 16–24 mg daily have been more effective than tramadol 300 mg daily in pain following knee surgery [Citation13]. The clinical trials published so far mostly comparative clearly document the efficacy of lornoxicam as a potent analgesic with excellent anti-inflammatory properties in a range of painful and/or inflammatory conditions including postoperative pain [Citation14] and rheumatoid arthritis [Citation12]. In accordance with these clinical trials, the analgesic effect of iv. lornoxicam in the current study was clinically evident by prolonged time to analgesic request, reduced analgesic consumption and lower pain scores in the first 24 h postoperative.
In this study, the selected dose of lornoxicam was based on several clinical trials that found that a preoperative dose of 8 mg was efficient for postoperative analgesia [Citation14–Citation19]. Pediatric doses for iv. lornoxicam ranged from 0.25 to 0.3 mg/kg [Citation20–Citation22], and as the mean body weight in this study ranged from 32.10 to 33.70 kg, we thought that fixing a dose of 8 mg would also be beneficial in the age group studied.
The peritonsillar drug infiltration had been used successfully in previous clinical trials. Drugs given by this route such as pethidine [Citation23], ketamine [Citation24], tramadol [Citation25], and bupivacaine [Citation26] provided a long lasting and satisfactory postoperative analgesia compared with their iv. counterparts and the placebo. In contrast to these trials, we demonstrated that the analgesic efficacy of peritonsillar lornoxicam was unsatisfactory. The postoperative pain scores were close to the placebo group. Moreover, the highest pain score recorded at 24 h postoperative was in the lornoxicam infiltration group.
A recent clinical trial was compared between intravenous and peritonsillar lornoxicam in adult tonsillectomy and concluded that, preoperative peritonsillar lornoxicam is not superior to intravenous lornoxicam with comparable intraoperative blood losses [Citation27]. However, in the current study, we observed an increased intraoperative blood loss in the lornoxicam infiltration group. Measures to control bleeding from the tonsillar bed such as diathermy and surgical suturing were used frequently in patients in the infiltration group. Such techniques are commonly associated with increased postoperative pain and might be in part responsible for the reduced analgesic efficacy of peritonsillar lornoxicam.
Clinical research studies had demonstrated the analgesic efficacy of locally applied lornoxicam given; intraarticular [Citation13], locally administered before lumbar epidural anesthesia [Citation28] and in intravenous regional anesthesia for hand or forearm surgery [Citation29] with no effect on bleeding. However, for peritonsillar administration of lornoxicam, further studies are needed to investigate the safety and efficacy of such application specially in pediatric population.
NSAIDS and perioperative bleeding during tonsillectomy are still a focus of debate. NSAIDS inhibit cyclooxygenase, leading to inhibition of platelet thromboxane A2 (TX A2) production and platelet aggregation resulting in prolongation of bleeding time [Citation30]. As platelets play a central role in the coagulation process, some authors believe that NSAIDS should be contraindicated during tonsillectomy [Citation31,Citation32], while others report that they are a useful and safe addition to postoperative analgesia, without an increase in perioperative bleeding [Citation33,Citation34]. Intravenous lornoxicam has been used in adults undergoing hysterectomy [Citation35], major abdominal surgery [Citation14], tonsillectomy [Citation27], and knee arthroscopy [Citation13], without any evidence of major perioperative bleeding. However, these research trials were focused on analgesic efficacy and were not powered to detect differences in the frequency of perioperative bleeding. Moreover, bleeding assessment was done subjectively without the use of specific platelet function assessment tests.
The bleeding time which has long been a stable of hemostasis testing has been dropped from the test menu at many laboratories. In its place, tests such as platelet aggregometry, lumiaggregometry, platelet function analyzer-100, and more recently flow cytometry are increasingly used to monitor patients with possible bleeding disorders [Citation36]. Although being expensive, they allow for quantitative and qualitative assessment of some platelet functions.
In the current study, the percentages of invitro platelet aggregation triggered by adenosine diphosphate, collagen, and arachidonic were maximally inhibited 2 h after iv. 8 mg lornoxicam administration by 45.37%, 37.04%, and 36.31%, respectively, and nearly returned to baseline values within 24 h postoperative. Felfernig et al., in their study, demonstrated that a single preoperative dose of 16 mg iv. lornoxicam significantly inhibited platelet aggregation (assessed by whole blood impedance aggregometry), by 85%, and that inhibition lasted for at least 8 h after lornoxicam application [Citation37]. Another study compared the effect of rofecoxib (a selective NSAID) and three non-selective NSAIDS; acetylsalicylic acid, diclofenac, and lornoxicam, on platelet function measured by CD62 P selectin expression by using flow cytometry. They concluded that platelet function was significantly inhibited by acetylsalicylic acid, diclofenac, and lornoxicam but not by rofecoxib [Citation38].
In this study, firstly, although maximal platelet inhibition was observed in intravenous lornoxicam group, complains from increased intraoperative bleeding were more in the infiltration group, suggesting a direct local effect of peritonsillar lornoxicam as a causative factor rather than systemic absorbtion.
Secondly, the inhibition in platelet aggregation presented in intravenous group did not result in increased bleeding episodes. A future research question arises about the relation between the level of platelet aggregation inhibition and clinical presentation [Citation39].
Thirdly, patients in the placebo controls showed mild significant reductions in platelet functions. This was in contrast with several previous adult studies that suggested that perioperative stress leads to a hypercoagulable state closely related to the neuroendocrine stress response [Citation40]. This augmentation in platelet function during stress may be mediated through adrenergic activation of α2 receptor subtypes on platelet membrane [Citation41] or an endocrine-induced increase in platelet cytoplasmic tyrosine kinase which is responsible for the activation of glycoprotein IIb–IIIa receptors [Citation40]. In this study, the small sample size, age group selected, operation type, and the routine intraoperative administration of iv. dexamethasone might be responsible for these conflicting results.
Therefore, further studies are required to investigate the safety of lornoxicam in this respect, and lornoxicam should be avoided for tonsillectomy in patients where increased blood loss poses a special risk as in hemorrhagic diathesis [Citation7].
In the current study, no patient complained from gastrointestinal adverse effects. These results support the facts that lornoxicam combines the high therapeutic potency of oxicams with a high tolerability and an improved gastrointestinal toxicity profile as compared to the other oxicams [Citation12,Citation42].
In conclusion; the intraoperative preincisional intravenous lornoxicam administration enhanced postoperative analgesia after tonsillectomy in children. In comparison, the analgesic efficacy of locally applied lornoxicam was inferior to the intravenous administration and was associated with an increased incidence of intraoperative bleeding. For conclusive platelet aggregometry results, a powered large sample sized studies should be undertaken.
Financial support
Support was provided solely from departmental resources.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- P.CastellanoJ.A.Lo’pez-Esca’mezAmerican Society of Anesthesiology classification may predict severe post-tonsillectomy haemorrhage in childrenJ Otolaryngol322003302307
- S.KrishnaL.F.HughesS.Y.LinPostoperative hemorrhage with nonsteroidal anti-inflammatory drug use after tonsillectomy: a meta-analysisArch Otolaryngol Head Neck Surg129200310861089
- S.MoinicheJ.RomsingJ.B.DahlM.R.Trame’rNonsteroidal anti-inflammatory drugs and the risk of operative site bleeding after tonsillectomy: a quantitative systemic reviewAnesth Analg9620036877
- A.J.SouterB.FredmanP.F.WhiteControversies in the perioperative use of nonsteroidal anti-inflammatory drugsAnesth Analg79199411781190
- N.VadiveluS.MitraD.NarayanRecent advances in postoperative pain managementYale J Biol Med8320101125
- G.HitzenbergerF.Radhofer-WelteF.TakacsD.RosenowPharmacokinetics of lornoxicam in manPostgrad Med J66Suppl. 41990S22S27
- N.M.SkjodtN.M.DaviesClinical pharmacokinetics of lornoxicam. A short half-life oxicamClin Pharmacokinet341998421428
- J.A.AldreteD.KroulikA postanesthetic recovery scoreAnesth Analg491970924934
- J.BauerClinical laboratory method9th ed.1982Mosby Co.St. Louis
- J.BergH.FellierT.ChristophJ.GrarupD.StimmederThe analgesic NSAID lornoxicam inhibits cyclooxygenase (COX)-1/-2, inducible nitric oxide synthase (iNOS), and the formation of interleukin (IL)-6 invitroInflamm Res4871999369379
- B.CanduzH.AktugO.MavioğluY.ErkinO.YilmazY.UyanikgilH.KorkmazM.BakaEpidural lornoxicam administration—innocentJ Clin Neurosci14102007968974
- S.Radhofer-WelteX.RabassedaLornoxicam, a new potent NSAID with an improved tolerability profileDrugs Today (Barc)36120005576
- M.ErenK.KoltkaG.Köknel TaluM.AşikS.Ozyal'çćinComparison of analgesic activity of intraarticular lornoxicam, bupivacaine and saline after knee arthroscopyAgri20420081722
- Y.KaramanE.KebapciA.GurkanThe preemptive analgesic effect of lornoxicam in patients undergoing major abdominal surgery: a randomized controlled studyInt J Surg632008193196
- M.SenerC.YilmazerI.YilmazEfficacy of lornoxicam for acute postoperative pain relief after septoplasty: a comparison with diclofenac, ketoprofen, and dipyroneJ Clin Anesth202008103108
- K.InanogluS.GorurC.O.AkkurtO.E.GuvenA.KararmazThe analgesic efficacy of preoperative versus postoperative lornoxicam in varicocele repairJ Clin Anesth192007587590
- O.SapolyaB.KaramanhogluD.MemisAnalgesic effects of lornoxicam after total abdominal hysterectomyJ Opioid Manage32007155159
- M.SenerC.YilmazerI.YilmazE.CaliskanA.DonmezG.ArslanPatient-controlled analgesia with lornoxicam vs. dipyrone for acute postoperative pain relief after septorhinoplasty: a prospective, randomized, double-blind, placebo-controlled studyEur J Anaesthesiol252008177182
- B.IşikM.Arslanö.ÖzsoylarM.Ak'abayPreoperative lornoxicam versus tramadol on postoperative pain and adverse effects in adult tonsillectomyAgri212009113120
- S.T.VergheseR.S.HannallahAcute pain management in childrenJ Pain Res1532010105123
- Li Xiuzel, Tan Ling. A comparison of analgesic efficacy and safety of tramadol and lornoxicam for postoperative pain relief in children. Sichaun Med J; 2007-07.
- Fu Yuezhen, Jin Quanying, Gu Zhiqing. Clinical observation of postoperative patient controlled intravenous analgesia in 612 children. J Pract Diag Ther; 2007-11.
- R.NikandishB.MaghsoodiS.KhademiS.MotazedianR.KaboodkhaniPeritonsillar infilteration with bupivacaine and pethidine for relief of posttonsillectomy pain: a randomized double-blind studyAnaesthesia63120082025
- A.HonarmandM.R.SafaviM.JamshidiThe preventive analgesic effect of preincisional peritonsillar infilteration of two low doses of ketamine for postoperative pain relief in children following adenotonsillectomyPaediatr Anaesth1862008508514
- M.B.UgurM.YilmazH.AltunkayaF.CinarY.OzerL.BederEffects of intramuscular and peritonsillar injection of tramadol before tonsillectomy: a double blind, randomized, placebo-controlled clinical trialInt J Ped Otorhinolaryngol722008241248
- S.OrntoftA.LongreenS.MoinicheJ.B.DhalA comparison of pre- and postoperative tonsillar infilteration with bupivacaine on pain after tonsillectomy. A pre-emptive effect?Anaesthesia4921994151154
- S.A.IsmailH.A.MowafiPreoperative peritonsillar lornoxicam infilteration is not superior to intravenous lornoxicam for pain relief following tonsillectomy in adultsEur J Anesthesiol2792010807811
- B.MusluB.UstaS.MusluA.YeşilayM.GözdemirH.SertR.DemircigluEffect of locally administered lornoxicam in the management of low back pain after lumbar epidural anesthesia: a double-blind, randomized, controlled studyMinerva Anestesiol7592009494497
- I.O.KolH.OzturkK.KaygusuzS.GursoyB.ComertC.MimarogluAddition of dexmedetomidine or lornoxicam to prilocaine in intravenous regional anesthesia for hand or forearm surgery: a randomized controlled studyClin Drug Investig2922009121129
- I.PowerW.A.ChambersI.A.GreerD.RamageE.SimonPlatelet function after intramuscular diclofenacAnaesthesia451990916919
- W.M.SplinterE.J.RhineD.W.RobertsC.W.ReidH.B.MacNeillPreoperative ketorlac increases bleeding after tonsillectomy in childrenCan J Anaesth431996560563
- A.SchmidtS.BjörkmanJ.AkesonPreoperative rectal versus paracetamol for tonsillectomy: effects on pain and blood lossActa Anaesthesiol Scand4520014852
- A.JeyakumarT.M.BrickmanM.E.WilliamsonNonsteroidal anti-inflammatory drugs and postoperative bleeding following adenotonsillectomy in pediatric patientsArch Otolaryngol Head Neck Surg13420082427
- S.A.MckeanM.S.LeeS.S.HussainComparative study of posttonsillectomy hemorrhage with the use of diclofenac versus dihydrocodeine for postoperative analgesia and review of the literatureJ Otolaryngol Head Neck Surg372008577581
- Z.Y.GongT.H.YeX.T.QinG.X.YuX.Y.GuoA.L.LuoPatient-controlled analgesia with lornoxicam in patients undergoing gynecological surgeryKe Xue Yuan Xue Bao2352001472475
- A.M.ZeidanP.A.KouidesM.A.TaraW.A.FrickePlatelet function testing: state of the artExpert Rev Cardiovasc Ther552007955967
- M.FelfernigA.SalatO.KimbergerP.GradisekM.R.MillerD.FelfernigPreemptive analgesia by lornoxicam- an NSAID-significantly inhibits perioperative platelet aggregationEur J Anaesthesiol2592008726731
- A.M.BlaicherH.T.LandsteinerO.Al-FalakiJ.ZwerinaI.VolfD.GruberM.ZimpferK.HoeraufAcetylsalicylic acid, diclofenac, and lornoxicam, but not rofecoxib affect platelet CD 62 P expressionAnesth Analg984200410821085
- Harrington RA, Kliman NS, Granger CB, Ohman EM, Berkowitz SD. Relation between inhibition of platelet aggregation and clinical outcome. Am Heart J 1998;136(4 pt 2 su):s43–50.
- B.A.RosenfeldN.FaradayD.CampbellHemostatic effects of stress hormone infusionAnesthesiology811994116126
- B.A.RosenfeldN.FaradayD.CampbellPerioperative platelet reactivity and the effects of clonidineAnesthesiology791993255261
- N.RawalK.KronerM.Simin-GeertsenC.HejlR.LikarSafety of lornoxicam in the treatment of postoperative pain: a post-marketing study of analgesic regimens containing lornoxicam compared with standard analgesic treatment in 3752 day-case surgery patientsClin Drug Investig30102010687697