Abstract
Dexmedetomidine a highly selective α2 agonist has become a frequently used drug in anesthesiologists’s armamentarium due to its sedative, anxiolytic, analgesic, neuroprotective and anesthetic sparing effects and a favorable side effect profile. Dexmedetomidine–lignocaine combination has been used recently to provide Bier’s block and was shown to improve quality of anesthesia, to reduce tourniquet pain and to reduce postoperative anesthetic requirement in patients undergoing forearm or hand surgeries. Hypotension and bradycardia are the commonly seen side effects. Only one case of dexmedetomidine skin allergy has been reported till date in literature. We present a case of dermatological allergy to dexmedetomidine, in a patient administered Bier’s block with dexmedetomidine–lignocaine combination for implant removal surgery of forearm.
1 Introduction
Bier’s block was first described in 1908 for anesthesia of hand and forearm and is a simple and reliable technique specially for day care surgeries. Lidocaine is the standard local anesthetic used in Bier’s block but many additives are now being used to decrease the time of onset of block, prolong the duration of block, decrease the tourniquet pain and to achieve postoperative analgesia [Citation1].
Recently α2 adrenergic agonists are commonly being used in anesthesia practice due to their sedative, analgesic, cardiovascular stabilizing effects and low incidence of side effects. They also prolong the LA-induced analgesia when used in regional blocks [Citation2,Citation3]. Addition of clonidine or dexmedetomidine to lignocaine in Bier’s block reduces the time of onset of block, improves the tolerance to tourniquet pain and decreases post-operative analgesic requirements [Citation4].
Dexmedetomidine, a highly selective α2 agonist, is eight times more selective for α2 adrenoceptors than clonidine. Bradycardia and hypotension are the commonly seen side effects and skin rash has been reported in one patient.
We report a case of a severe rash due to dexmedetomidine in a 25 years old male patient posted for elective surgery of removal of right radial plate under Bier’s block with combination of lignocaine–dexmedeomidine.
We obtained the patients’ permission for publishing this case.
2 Case description
A 25 years old, 50 kg American Society of Anaesthesiologists (ASA) Class I male patient was scheduled for elective surgery for removal of left radial plate. The plating was done one year ago for fracture left radius due to road traffic accident. The plating was performed under general anesthesia (propofol, vecuronium, isoflurane anesthesia) an year ago. Intravenous regional anesthesia with dexmedetomidine–lidocaine combination was planned.
A written informed consent was obtained from the patient. The patient had no previous exposure to local anesthetic. So sensitivity testing for lidocaine was done one day prior to surgery by an intradermal injection of 0.1 ml of 2% preservative-free plain lignocaine on the ventral aspect of forearm. There was no erythema or wheal and the test was non-reactive. An intravenous cannula 20G was inserted on the dorsum of non-operative hand and first dose of injection ceftriaxone 1 gm was administered on the morning of the day prior to surgery for peri-operative antibiotic coverage. On the night prior to surgery, tablet ranitidine hydrochloride 150 mg per oral and intravenous ceftriaxone 1 gm (second dose) was administered.
On the day of surgery, after confirming nil-by-mouth status, standard monitors including 5-lead electrocardiography, non-invasive blood pressure (NIBP) and pulse oximetry probe were used (Datex Ohmeda AESTIVA 5, GE Healthcare, Helsinki, Finland). A 22G intravenous (IV) cannula was inserted on the dorsum of the hand to be operated on, for administration of Bier’s block. Infusion of Ringer Lactate was started through the intravenous cannula present on the non-operative hand. Premedication was achieved with intravenous ondansetron 4 mg and intravenous ranitidine hydrochloride 50 mg and the third dose of antibiotic (intravenous ceftriaxone 1 gm) was administered.
A double tourniquet (Diamond Tourniquet, Industrial Electronic and Allied Products, Pune, India) was positioned on the upper aspect of the operative arm. The operative extremity was exsanguinated by elevation for 3 min and wrapping it with a 10 cm Esmarch bandage. The proximal tourniquet was inflated to 250 mm of Hg (systolic BP = 124 mm of Hg) and the Esmarch bandage was removed. Circulatory isolation of the operative hand was confirmed by absence of the radial pulse and disappearance of the pulse oximetry tracing.
The Bier’s block was achieved using preservative-free 0.5% lidocaine in the dose of 3 mg kg−1 [LOXICARD∗, NEON LABORATORIES LIMITED, Andheri(East), Mumbai, India.] i.e. for 50 kg patient, 7.5 ml 2% lidocaine diluted with saline to a total volume of 40 ml to which dexmedetomidine hydrochloride 0.5 μg kg−1 [DEXTOMID∗, NEON LABORATORIES LIMITED, Andheri(East), Mumbai, India] was added. The dexmedetomidine–lignocaine combination was administered slowly over one minute via the IV cannula on the operative limb.
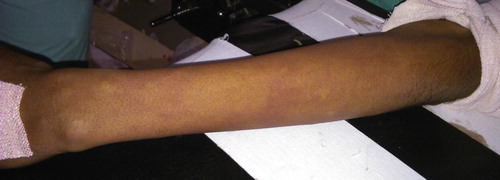
Approximately 90 s after the injection, a wheal and flare type of rash was noted in the operative limb. Such rash was not seen on any other aspect of the patient’s body (see photograph). Rash occurred 25–30 min after antibiotic injection and 90 s after administration of Bier’s block. Immediately injection hydrocortisone 100 mg was administered via the cannula on the operative limb. On questioning, the patient denied to the presence of any feelings of giddiness, nausea or breathlessness. Prophylactic injection hydrocortisone 100 mg was also administered via the cannula on non-operative limb. Oxygen supplementation was done via facemask at 5 l per minute. The patient’s vitals were closely monitored at one minute intervals. Vitals remained stable and bronchospasm, hypotension, bradycardia or arrhythmias were not observed. Approximately 10 min after this episode and 20 min after administration of Bier’s block, when the sensory and motor blocks were confirmed, the distal tourniquet was inflated to 250 mm of Hg, proximal tourniquet was deflated, intravenous cannula on the operative limb removed and surgery commenced.
The surgery proceeded uneventfully and was completed in 75 min. At that time, reduction in the allergic rash was noted but it was still present. There was no tourniquet pain and so the patient was kept under observation in the operating room. Keeping resuscitation equipment and drugs ready, the distal tourniquet was released at 120 min after administration of Bier’s block. At the time of release of tourniquet only minimal rash was present. The patient’s hemodynamic vitals remained stable.
Monitoring was continued in the operating room but signs of rash anywhere else on the body or signs of hemodynamic instability were not observed. The patient was shifted to the post-anesthesia care unit after one hour. The rash completely resolved 4 h after its appearance and patient was shifted toward after 24 h.
3 Discussion
Our patient, a 25 years old male, who had undergone fixation of left radius fracture an year ago under an uneventful general anesthesia using Propofol, vecuronium and isoflurane was scheduled for radial plate removal surgery. Decision to conduct surgery under intravenous regional anesthesia was taken. There was no history of exposure to local anesthesia in past. There was no history of any drug allergy in past.
We used 0.5% lidocaine in our patient. Sensitivity test for lidocaine done one day prior to surgery was non-reactive.
Dexmedetomidine was approved by the Food and Drug Administration in December 1999 for use in humans as a short-term medication (<24 h) for analgesia and sedation in the intensive care unit (ICU). The adverse effects of dexmedetomidine include hypotension, hypertension, nausea, bradycardia and atrial fibrillation. Most of the adverse events associated with dexmedetomidine use occur during or briefly after loading dose of the drug [Citation5].
Jaakola et al. demonstrated the analgesic efficacy of dexmedetomidine in human tourniquet pain. In their study, a single IV dose of fentanyl and dexmedetomidine (0.25, 0.5, and 1 μg/kg) was administered in healthy volunteers. They found that dexmedetomidine clearly demonstrated an analgesic effect in the tourniquet test [Citation6].
Dilek Memis et al. were the first to demonstrate clinically that the addition of 0.5 μg/kg dexmedetomidine to lidocaine for IVRA improves quality of anesthesia and improves intra-operative-postoperative analgesia without causing side effects [Citation4].
M.A. Abosedira in a study concluded that dexmedetomidine–lidocaine mixture provided better quality of anesthesia, tourniquet tolerance and operative and postoperative analgesia. The author also reported an increase in post-cuff deflation sedation in dexmedetomidine–lidocaine patients as compared to clonidine–lidocaine mixture [Citation1].
We decided to add dexmedetomidine 0.5 μg per kg to 0.5% lidocaine in Bier’s block to improve quality of intra-operative anesthesia and to reduce postoperative analgesic requirement.
The proximal tourniquet was inflated to 250 mmHg and dexmedetomidine–lidocaine mixture was slowly injected through the intravenous cannula on the operative limb. A wheal and flare type of rash was encountered along the course of the vein on the operative limb approximately 90 s after the injection of Bier’s block.
We immediately administered intravenous hydrocortisone through the same cannula. We also administered prophylactic hydrocortisone through the cannula on contralateral hand. The mechanism of action of hydrocortisone is suppression of the inflammatory response by limiting the recruitment of inflammatory cells at the local sites [Citation7]. We were unsure about the exact effect produced by such an injection of hydrocortisone in Bier’s block. It was nevertheless administered as it was the only possible step to reduce the inflammatory response at the site. Systemic hydrocortisone was administered to counteract the inflammatory mediators, if any, released after deflation of tourniquet.
Ludwig et al. [Citation8] observed a similar wheal and flare rash encompassing 60% of body surface area in an intubated patient, injured in a motor vehicle collision, 4 h after starting dexmedetomidine infusion (0.2 μg/kg/h). The rash completely resolved 48 h after discontinuation of dexmedetomidine infusion.
Okabe et al. [Citation9] reported a case of drug fever caused by dexmedetomidine hydrochloride infusion. The patient was transferred to the intensive care unit with an abdominal aortic aneurysm rupture. After initiation of sedation with dexmedetomidine hydrochloride, he developed pyrexia of more than 39 °C. This symptom improved rapidly 7 h after stopping dexmedetomidine hydrochloride. Other possible causes (such as infection) were sequentially eliminated.
When Naranjo adverse drug reaction probability score was calculated, our score of 5 points indicates that there is a ‘probability’ of dexmedetomidine causing this reaction [Citation10]. We have already ruled out the other possible agents causing such rash namely, lidocaine, antibiotic or premedication.
This is the second case report of dermatological allergic response to dexmedetomidine.
We conclude that anesthesiologists should be aware of allergic dermatologic reactions to dexmedetomidine specially in the present setup, when it is being widely used as an intravenous analgesic and as an additive in regional anesthesia (see ).
Conflicts of Interest
None.
Notes
Available online 29 March 2014
References
- M.A.AbosediraAdding clonidine or dexmedetomidine to lidocaine during Bier’s block: a comparative studyJ Med Sci872008660664
- D.B.MurphyC.J.L.McCartneyV.W.S.ChanNovel analgesia adjuncts for brachial plexus block: a systemic reviewAnesth Analg90200011221128
- T.KamibayashiM.MazeClinical uses of alpha-2-adrenergic agonistsAnesthesiology93200013451349
- Memis¸DilekTuranAlparslanluBeyhan KaramanlıogPamukc¸uZaferKurtImranAdding dexmedetomidine to lidocaine for intravenous regional anesthesiaAnesth Analg982004835840
- H.Ralph GertlerBrownCleightonDonald H.MitchellErin N.SilviusSilvius. Dexmedetomidine a novel sedative-analgesic agentProc(Bayl Univ Med Cent)14120011321
- M.L.JaakolaT.Ali-MelkkilaJ.KantoDexmedetomidine reduces intraocular pressure in intubation responses and anaesthetic requirements in patients undergoing ophthalmic surgeryBJA681992570575
- J.S.GoodwinD.AtluruS.SierakowskiE.A.LianosMechanism of action of glucocorticosteroids. Inhibition of T cell proliferation and interleukin 2 production by hydrocortisone is reversed by leukotriene B4J Clin Invest774198612441250
- K.LudwigM.SorrellP.LiuSevere rash associated with dexmedetomidine use during mechanical ventilationPharmacotherapy2942009479481
- OkabeTadashiTakedaShinhiroAkadaShinjiHongoTakashiSakamotoAtsuhiroPostoperative intensive care unit drug fever caused by dexmedetomidineAnesth Analg108200915891591
- C.A.NaranjoU.BustoE.M.SellersP.SandorI.RuizE.A.RobertsA method for estimating the probability of adverse drug reactionsClin Pharmacol Therap301981239245