Abstract
Background
Recently dexmedetomidine had been successfully used in conscious (moderate) sedation as a good competitive to popular agent (midazolam). Different concentrations of ketamine and propofol combinations (ketofol) were used for procedural sedation and analgesia.
Objectives
The study was conducted to compare two techniques of moderate sedation for patients undergoing ERCP, using either dexmedetomidine or ketofol as regards hemodynamic, sedation, pain, respiratory effect, recovery time, patients’ and endoscopists’ satisfactions, and complications during and after the procedure.
Patients and methods
Fifty patients were randomly allocated in one of two groups; dexmedetomidine group D (n = 25) received 1 μg/kg i.v. bolus over 10 min followed by 0.5 μg/kg/h or ketamine/propofol (ketofol) group KP (n = 25) received 1 mg/kg i.v. bolus followed by 50 μg/kg/min. The level of sedation was adjusted to achieve a Ramasy sedation scale (RSS) score of 4 in both groups of patients. Mean arterial pressure (MAP), heart rate (HR), peripheral oxygen saturation (SPO2), and facial pain score (FPS) were compared. Time to achieve RSS, modified Aldrete’s score (MAS) of 9–10 and the related complications were compared between groups. Patients’ and endoscopists’ satisfactions were compared. Total amount of rescue sedation was recorded.
Results
After loading dose HR and MAP were significantly lower in group D as compared with group KP (p < 0.05). HR was significantly lower in group D during the recovery (p < 0.05). No significant difference between both groups as regards time to achieve RSS, MAS, FPS and total dose of rescue sedation. Personnel restraint was significantly lower in group KP (8% versus 20%) than in group D. Endoscopists’ satisfaction was significantly higher in group KP than D group (92% and 80%) respectively.
Conclusion
Ketofol (1:1) provided better hemodynamic stability than dexmedetomidine and standard alternative to it in moderate sedation during ERCP.
1 Introduction
There were many challenges during moderate sedation for ERCP in endoscopy unit; as remote location, less familiar area, semi prone position, lengthy procedure and shared airway. It should ensure immobility, sufficient analgesia, avoid coughing or gagging and allow patient comfort to avoid any complication as perforation or peritonitis [Citation1]. Moderate sedation sufficient dose should not suppress the airway protective reflexes and monitoring methods should be administered [Citation2]. ERCP (diagnostic or therapeutic) was complicated and lengthy procedure. It needed moderate, deep sedation, and even general anesthesia. The level of sedation as well as the choice of sedative based on type of procedure and patient characteristics. Diagnostic ERCP procedures involving bile or pancreatic duct as papillotomy, and dilation of ampulla of vater needed for moderate sedation/analgesia but more complicated and lengthy procedure as lithotripsy, stone removal and implantation of the stent needed for deep sedation and even general anesthesia (GA) . Patient selection according to ASA classification and the history of long term used narcotics, benzodiazepines or any neuropsychiatric medications are very important in choosing the sedative agent and the level of sedation [Citation3]. In patients receiving moderate sedation during ERCP, pain and discomfort during and immediately after ERCP were experienced by one third to one half of the patients due to inadequate level of sedation, as well as inadequate selection of sedative agent against type of procedure and patient physical and mental status. Incremental doses of midazolam lead to unintended deep level of sedation as well as hypotension and desaturation [Citation4]. There was higher failure rate due to premature termination of ERCP because of inadequate sedation [Citation5]. There were many trials to improve the patient’s comfort and the safety. General anesthesia outside the operating room had cost burden and increased risk of complication and still remained controversial [Citation6]. Dexmedetomidine is a highly selective α2 agonist that has sedative, analgesic, anxiolytic, and amnesic effects without a significant respiratory depression [Citation7]. It displays a dose-dependent blood pressure response. It has a sympatholytic effect through decreasing the concentration of norepinephrine which in turn decreases the heart rate and blood pressure [Citation8]. Ketamine is an NMDA receptor antagonist and has also been found to bind to opioid and sigma receptors. It is phencyclidine derivatives and is classified as a dissociative sedation with fairly rapid onset and duration with little or no respiratory and cardiovascular depression. It causes amnesia and analgesia but its use as a single sedative agent has been limited because of its emergence reactions [Citation9]. Propofol is non-opioid, non-barbiturate, popular sedative, hypnotic agent with rapid onset, short duration of action. It has undesirable side effects as cardiovascular and respiratory depressions which need cardiopulmonary support [Citation10]. The combination of ketamine and propofol (ketofol) with low doses of each appeared with a better hemodynamic and respiratory stability. Ketofol is physically compatible and chemically stable and it can be stored at room temperature and under light [Citation11,Citation12]. Ketamine is adding analgesia to propofol sedation, while vomiting and hallucination induced by ketamine are countered by propofol antiemetic and hypnotic properties [Citation13]. There was a single study on the use of ketofol for moderate sedation in ERCP but with different concentration of ketofol [Citation14]. The aim of the study was to compare between two regimens of moderate sedation; dexmedetomidine versus ketofol in ERCP as regards hemodynamic, sedation, pain, respiratory effect, recovery time, patients’ and endoscopists’ satisfactions, and complications during and after the procedure.
2 Patients and methods
A prospective double-blind randomized study was carried out in King Abdul-Aziz hospital, Saudi Arabia; March 2013 to March 2014. Approval of ethical committee and written informed consents from patients were obtained. Patients scheduled for elective diagnostic and therapeutic ERCP. The study enrolled 50 patients were ASA physical classifications I and II, aged 18–60 years, and from both sexes. We excluded patients who had ASA physical statuses III and IV, allergy to any drug used in the study, history of sedative and narcotic analgesic drugs, cardiovascular diseases as hypertension, mitral stenosis, aortic stenosis, arrhythmia, or congestive heart failure, liver or renal insufficiency, diabetes, seizures, pregnancy, and morbid obese or uncooperative patient. Preoperative assessment including general, systemic examination, and routine laboratory investigation was done. Patients were randomly allocated by the “sealed envelope technique” to one of two groups by 1:1 ratio. They received either of the following two regimens: dexmedetomidine group D (n = 25) or ketofol group KP (n = 25). On arrival in endoscopy unit the sealed envelope opened and the written regimen applied. Antecubital venous access secured on nondominant hand by 20 G intravenous cannula, ringer lactate drip started by 8 ml/kg/h and nasal cannula by 4 L/min o2. Basal HR, MAP, and spo2 were recorded. The study drugs were prepared by an assistant who was not involved in the study. He covered all syringes and infusion sets by silver paper due to different colors. Both bolus and maintenance doses were given using syringe pump (Abbott-life care 5000). The groups were similar in respect to time of procedure by the same endoscopist. For dexmedetomidine group D; (Precedex 200 μg/2 ml; Abbott) which prepared as 2 ml Precedex plus 18 ml normal saline total volume 20 ml (1 ml = 10 μg Precedex); patients received loading dose of 1 μg/kg i.v. over 10 min followed by 0.5 μg/kg/h infusion until achieving RSS of 4. In ketofol group KP; patients received ketofol which prepared as 2 ml ketamine (50 mg/ml) and 10 ml propofol 1% (10 mg/ml) plus 8 ml normal saline. The mixture was 20 ml by 1:1 and each ml contained 5 mg for ketamine and 5 mg for propofol. Patients received 1 mg/kg over 10 min and followed by 50 μg/kg/min till achieving RSS of 4 (). The level of sedation assessed at 1–3 min interval and the infusion rate adjusted to achieve RSS of 4. The anesthesiologist who gave the drugs and assessed parameters was blinded to the randomization process and to the drugs of study but he can adjust the infusion rate till achieving RSS of 4. Adjustment of group D was 0.1–0.2 μg/kg/h and for group KP was 25 μg/kg/min. Dexmedetomidine 20 ml syringe was labeled as bolus and infusion 1 and ketofol 20 ml syringe was labeled as bolus and infusion 2. Time to achieve RSS of 4 in both groups was recorded. HR, MAP, and SpO2 were also recorded following the loading dose and every 5 min until completion of the procedure. The FPS (0–10) to evaluate pain at 5 min interval during the procedure () and in the recovery till MAS reached 9–10 (). During the procedure if patient required personnel restraint, either patient or endoscopist was uncomfortable or FPS more than 5, the rescue IV sedation was provided with propofol in top up increment dose of 10 mg and total dose was recorded. During the procedure, any of the following complications were noted, recorded and treated accordingly: oxygen desaturation was considered when SpO2 less than 92% for more than 10 s. Apnea was defined as not having a spontaneous breathing for at least 20 s. Both were managed by supporting airway and/or assisting ventilation. Bradycardia was considered when HR was less than 60 beats/min and managed with atropine 20 mcg/kg i.v. Hypotension was considered when MAP decreased by >20% of the baseline MAP and managed by fluid bolus or vasopressors. Any cough or gagging was noted and recorded. The study drug infusion discontinued at the end of the procedure. During the recovery, the recovery nurse was blinded to study medication and the following parameters were recorded: the recovery time was considered from discontinuation of the infusion till achieving MAS 9-10 and during that time HR, MAP, and SpO2 were recorded at 5 min interval. Postoperative nausea and vomiting (PONV), apnea, pain and agitation were recorded and managed accordingly. Patients’ and endoscopists’ satisfactions were assessed using satisfaction score (4 = excellent, 3 = good, 2 = fair, and 1 = bad). Patient and endoscopist considered satisfied with good and excellent scores and % of satisfaction was calculated in both groups.
Table 1 Ramsay Sedation Scale.
Table 2 Modified Aldrete’s score.
3 Statistical analysis
A power analysis for sample size suggested a minimum 24 patients in each group with a mean of 94.4 mm Hg of MAP in group PK, and 75.5 mm Hg of MAP in group D and SD 2.7 with (α = 0.05) gave statistical power 98.8%. We included 50 patients in both groups in the study. Data were statistically described in terms of mean ± standard deviation (±SD), or frequencies (number of cases) and percentages when appropriate. Numerical data between both groups were done using Student’s t-test for independent samples. Categorical data were compared by Chi-square test. P values less than 0.05 were considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
4 Results
There were no significant differences in either the demographic data, duration of the procedure or total doses of rescue sedation (propofol) between the two groups. (). There were no significant differences in either preoperative or recovery vital signs except patients in group D had statistically significant (P < 0.05) lower HR (74 ± 4.3 beat/min) and slight tachycardia (91.3 ± 7.9 beat/min) in group KP during recovery (). Total dose of ketamine or propofol in ketofol used during the procedure was (130 ± 0.5 mg) of each. There was no increment dose used in both groups. Total doses of rescue sedation of propofol were 27.2 ± 1.7 and 24.5 ± 2.8 mg in group D and KP respectively with no significant difference between both groups p > 0.05.
Table 3 Demographic characteristic, duration of procedure and total dose of rescue sedation.
Table 4 Vital signs preoperative and during recovery.
Patients in group D had statistically significant (P < 0.05) lower HR and MAP after the loading dose at 5, 10, 15, 20, 25, and 30 min during ERCP procedure ( and ). There were no significant differences in SpO2. The time to achieve RSS (12.4 ± 1.1 min) and (13.2 ± 0.5 min) or the time to achieve MAS ⩾ 9 (11.4 ± 0.5) min and (12.5 ± 1.8 min) with (p > 0.05) between both groups respectively () .
Table 5 Changes of HR between both groups during the procedure.
Table 6 Changes of MAP between both groups during the procedure.
Table 7 Time to achieve RSS and MAS.
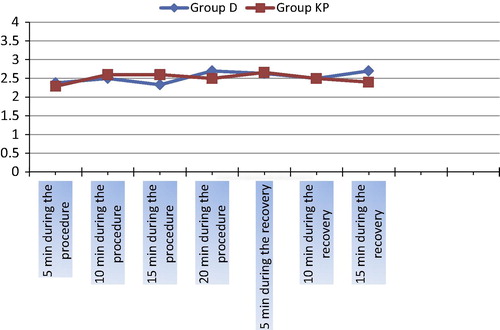
Patients in group D showed higher incidence of restlessness (restraint) than group KP (8%) and (4%) respectively but patients in group KP showed higher incidences of PONV (16%) and agitation (8%) as compared with group D (8%) and (0%) respectively (). Both groups had similar FPS (p > 0.05) during ERCP and recovery (). Both patients’ and endoscopists’ satisfaction scores were higher in group KP (92% and 92%) as compared with group D (88% and 80%) with (P > 0.05) and (p < 0.05) respectively ().
Table 8 Complications during the procedure and recovery.
Table 9 Patients’ and endoscopists’ satisfactions score.
5 Discussion
The goals of the conducted study were to provide adequate steady state of sedation level while maintaining airway reflex, maintain cardiovascular and respiratory status, minimize side effects and pain and ensure patient comfort [Citation15]. The current study compared the efficacy and safety of two different regimens of moderate sedation. The dose of dexmedetomidine used in the study was similar to previous study [Citation16]. The ketofol dose was different and new to other studies which investigated the best admixture dose. The most of studies used ketofol as induction agent for short procedures or in deep sedation but no study used it in moderate sedation. They concluded that combination 4:1 of propofol/ketamine contributed adequate deep sedation and analgesia without hemodynamic and respiratory depression or psychotomimetic side effects [Citation17]. The current study used ketofol admixture 1:1 and continuous infusion to maintain adequate moderate sedation which monitored by RSS. In our study there was no event of hypotension or bradycardia observed in both regimens. Patients in group D showed lower HR and MAP and these results were in accordance with previous studies [Citation16,Citation18]. Patients in ketofol group showed more stability in HR and MAP because propofol/ketamine combination was thought to act by antagonizing the side effect which was consistent with previous study [Citation19]. They concluded that minimal changes observed in MAP and HR may be dose related and because sympathomimetic action of ketamine was effective in countered action of the hemodynamic depression of propofol and there was no occurrence of profound tachycardia or bradycardia in any group. These findings suggested that ketofol had clinical advantages over dexmedetomidine as regards controlling hemodynamic variability. In the current study there was no event of apnea or desaturation in both groups which matched with previous trials for both dexmedetomidine and ketofol [Citation20,Citation16]. In current study there was slight longer time to achieve RSS of 4 in group KP than group D (13.2 ± 0.5 min and 12.4 ± 1.1 min respectively) but it was still in the acceptable range and due to slow onset of action of ketamine. These results matched with previous study which compared ketofol with ketamine for sedation time and found that ketamine had 16 min sedation time versus 13 min for ketofol [Citation21]. In our study the recovery time of group D was shorter than group KP (11.4 ± 0.5 min and 12.5 ± 1.8 min respectively) and this was accepted. Slower clearance of ketamine was probably responsible for this [Citation19]. We used FPS for pain assessment during sedation because there was difficulty to communicate or ask the patient about pain score during sedation so we depended on patient’s face expressions. We also used it during the recovery because some patients were uncooperative to use VAS and they did not understand it. Both sedation drugs in our study provided low FPS during procedure and recovery. The rescue sedation of propofol was given only to reduce movement and personnel restraints with no statistical significant differences between both groups as regards rescue sedation or personnel restraints and there was no rescue analgesic in both groups. These results matched with Rapeport et al. who found ketofol 1:1 infusion used in conjunction with regional anesthesia was safe and effective in high risk patients and provided adequate sedation [Citation22]. In the recovery four patients (16%) in ketofol group showed PONV and emergence reactions by (8%) and these results accepted with higher dose of ketamine (112 ± 0.5 mg) which was countered acted by antiemetic and sedative effects of propofol and still lower than incidence rate of ketamine alone. Emergence reactions were 50% in adults which had been reported by others [Citation23–Citation25]. In the current study ketofol group had higher satisfaction scores of both patients and endoscopist. Our findings mismatched with Dere et al. results [Citation26]. They found high satisfaction for dexmedetomidine when compared with midazolam. Our findings were most probably due to fewer personnel restraints in ketofol group versus group D (4% versus 8%) respectively. Many studies compared dexmedetomidine versus midazolam in ERCP or colonoscopy [Citation26,Citation27]. They concluded that it was more efficient in hemodynamic and respiratory status with high satisfaction scores. We concluded that in our study but with less satisfaction scores. Andolfatto et al. used ketofol mixture (1:1) for emergency procedural sedation and analgesia by low dose ketamine 0.25 mg/kg and they found adequate sedation, relative high pain score and low unpleasant emergence [Citation2]. It explained by low dose of ketamine which provided less analgesia. In our study increased ketamine dose provided adequate analgesia and also still lower unpleasant emergence. Weatheral et al. used ketofol (1:1) by 1 mg/kg with regional anesthesia for orthopedic procedure and they found good safety margin for this combination with no episode of cardiovascular or respiratory compromise was documented [Citation28]. We used combination to add synergism and decrease unwanted side effects. Ketofol as a combination associated with improved hemodynamic when compared to standard midazolam, fentanyl or even dexmedetomidine sedation. Ketofol had been proved to be safe, effective and reliable in adults and children [Citation29]. Tosun et al. compared propofol 1.2 mg/kg and ketamine 1 mg/kg in ratio 1.2:1 versus propofol/fentanyl in the same ratio and both combinations provided effective sedation and analgesia during dressing changes in burnt patients in agreement with our results [Citation30]. Our results suggested that our combination propofol/ketamine 1:1 was more suitable in moderate sedation during ERCP (all diagnostic and therapeutic as stone removal and stent implantation). We used ketofol and dexmedetomidine by adjusted doses to ensure moderate level of sedation without affecting the hemodynamic and respiration. We used ketofol to ensure moderate sedation without unintended deep level of sedation to avoid its complication outside OR. The doses of two drugs were suitable in moderate sedation and effective in diagnostic and even some therapeutic ERCP with good selection of the patients to ensure safety.
6 Conclusion
Propofol/ketamine (1:1) bolus and modified infusion rate provided adequate sedation, with hemodynamic and respiratory stability, and accepted few side effects. It appeared to be safe with higher satisfaction scores for patients and endoscopists. It was a good competitive to standard sedative as dexmedetomidine in moderate sedation during ERCP.
7 Limitations of this study
The blinded study was difficult due to different colors of medications but it solved by sliver paper. The study included small group of patients but it was according to the sample size calculation. Further studies needed to increase the number of patients with the different concentrations of ketofol.
Conflict of Interest
We have no conflict of interest to declare.
Notes
Available online 29 November 2014
References
- D.R.LichtensteinS.JagannathT.H.BaronM.A.AndersonS.BanerjeeJ.A.DominitzSedation and anesthesia in GI endoscopyGastrointest Endosc6852008815826
- Y.DemiraranE.KorkutA.TamerY.IlknurK.BuketS.GulbinThe comparison of dexmedetomidine and midazolam used for sedation of patients under upper endoscopy: a prospective, randomized studyCan J Gastroenterol2120072529
- G.A.PaspatisG.TriboniasK.ParaskevaLevel of intended sedationDigestion8220108486
- S.M.JeurninkE.SteyerbergE.KuipersP.SiersemaThe burden of endoscopic retrograde cholangiopancreatography (ERCP) performed with the patient under conscious sedationSurg Endosc26201222132219
- K.RaymondosB.PanningI.BachemM.P.MannsS.PiepenbrockP.N.MeierEvaluation of endoscopic retrograde cholangiopancreatography under conscious sedation and general anesthesiaEndoscopy342002721726
- T.CookE.C.BehringerJ.BengerAirway management outside the operating room: hazardous and incompletely studiedCurr Opin Anaesthesiol252012461469
- D.S.CarolloB.D.NossamanU.RamadhyaniDexmedetomidine: a review of clinical applicationsCurr Opin Anaesthesiol212008457461
- A.ParisP.H.TonnerDexmedetomidine in anesthesiaCurr Opin Anaesthesiol182005412418
- M.G.RobackJ.E.WathenL.BajajJ.P.BothnerAdverse events associated with procedural sedation and analgesia in a pediatric emergency department: a comparison of parenteral drugsAcad Emerg Med122005508513
- G.A.CotéR.M.HovisM.A.AnsstasL.WaldbaumR.R.AzarD.S.EarlyIncidence of sedation-related complications with propofol use during advanced endoscopic proceduresClin Gastroen Hepatol82010137142
- Z.MorseK.SanoT.KanriEffect of propofol-ketamine admixture in human volunteersPac Health Dialog1020035154
- L.A.TrisselD.L.GildertJ.F.MartinezCompatibility of propofol emulsion with selected drugs during simulated Y – site administrationAm J Health Syst Pharm54199712871292
- E.WillmanG.AndolfattoA prospective evaluation of ketofol for procedural sedation and analgesia in the emergency departmentAnn Emerg Med4920132330
- H.RihamE.S.WaelKetamine/propofol versus fentanyl/propofol for sedating obese patients undergoing endoscopic retrograde cholangiopancreatography (ERCP)Egypt J Anesth292013207211
- M.T.AouadA.R.MoussaC.M.DagherAddition of ketamine to propofol for initiation of procedural anesthesia in children reduces propofol consumption and preserves hemodynamic stabilityActa Anaesthesiol Scand522008561565
- N.KilicS.SahinH.AksuB.YavascaogluConscious sedation for endoscopic retrograde cholangiopancreatography: dexmedetomidine versus midazolamEurasian J Med4320111317
- M.DaabisM.ElsherbinyR.AlotibiAssessment of different concentration of ketofol in procedural operationBMJ2120092731
- J.A.AlhashemiDexmedetomidine vs. midazolam for monitored anesthesia care during cataract surgeryBr J Anaesth962006722726
- A.AkinA.EsmaogluG.GulerR.DemirciogluN.NarinA.BoyaciPropofol and propofol/ketamine in pediatric patients undergoing cardiac catheterizationPediatr Cardiol262005553557
- S.R.ArainT.J.EbertThe efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedationAnesth Analg952002461466
- A.ShahG.MosdossyS.McLeodK.LehnhardtM.PeddleM.RiederA blinded, randomized controlled trial to evaluate ketamine/propofol versus ketamine alone for procedural sedation in childrenAnn Emerg Med5752011425433
- D.A.RapeportJ.W.MartyrL.P.WangThe use of ketofol (Ketamine/propofol admixture) infusion in conjunction with regional anesthesiaAnesth Intens Care3712009121123
- E.V.WillmanG.AndolfattoA prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency departmentAnn Emerg Med4920072330
- S.M.GreenB.KraussClinical practice guideline for emergency department ketamine dissociative sedation in childrenAnn Emerg Med442004460471
- C.R.ChudnofskyJ.E.WeberP.J.StoyanoffP.D.ColoneM.D.WilkersonD.L.HallinenA combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patientsAcad Emerg Med72000228235
- K.DereI.SuculluE.T.BudakS.YeyenA.I.FilizS.OzkanA comparison of dexmedetomidine versus midazolam for sedation, pain and hemodynamic control, during colonoscopy under conscious sedationEur J Anaesthesiol272010648652
- P.SethiM.SadikP.KumarN.GuptaDexmedetomidine versus midazolam for conscious sedation in endoscopic retrograde cholangiopancreatography: an open-label randomized controlled trialIndian J Anesth58120141824
- A.WeatherallR.VenclovasExperience with a propofol/Ketamine mixture for sedation during pediatric orthopedic surgeryPaediatr Anaesth2011201210091016
- N.SmischneyM.BeachR.LoftusT.M.DoddsM.D.KoffKetamine/propofol admixture (ketofol) is associated with improved hemodynamics as an induction agent: a randomized, controlled trialJ Trauma Acute Surg731201294101
- Z.TosunA.EsmaogluA.CoruhPropofol–ketamine vs propofol–fentanyl combinations for deep sedation and analgesia in pediatric patients undergoing burn dressing changesPediatr Anesth1820084347