Abstract
Progress made on local anesthetics controlled release formulation and their ability to induce motor and sensory block for a longer period of time brings significant advantages in clinical practice. The use of sustained release formulations provides analgesia for a long period of time with one administration, thus limiting the complications that can occur with conventional analgesia. Also, controlled release of a biologically active compound prevents overdosing, minimizing the side effects, especially cardiotoxicity, neurotoxicity and tissue lesions. Clinical use of liposomal formulation brings high impressive results in pain control and quick patient recovery. Increased patient comfort, reducing to half the hospital length of stay, and treatment costs are able to provide a higher level of healthcare.
1 Introduction
Pain management represents one of the major therapeutical goals in Intensive Care Unit [Citation1,Citation2]. Opioids represent the gold standard in pain control [Citation3]. However, their systemic administration is associated with unwanted and potentially severe side effects [Citation4] such as respiratory depression, drowsiness and sedation, nausea and vomiting, allergies and neutrophil disfunction [Citation1–Citation7].
Therefore, many sustained release systems were studied using local anesthetic agents [Citation8], liposomal bupivacaine being the most studied system [Citation9]. The present paper presents different liposomal systems used to obtain analgesia, and their applications in Anesthesia and Intensive Care Unit.
2 Liposomal drug delivery systems
Liposomes are spherical, small dimension nanovesicles consisting in a phospholipidical double layer [Citation10,Citation11]. Phospholipids have a polar group and two hydrophobic groups, usually an unsaturated fatty acid. Because in their constitution there is also a hydrophilic part, a number of water soluble active substances can be encapsulated without the drug structure being affected [Citation12,Citation13].
Currently, liposomes are of particular interest in clinical treatment due to its important properties (). Among these, the cell membrane biocompatibility and the ability to graft different ligands on their surface are the most important [Citation10,Citation14]. For this purpose, various controlled release systems based on liposomal nanovesicles have been developed. Several obstacles in drug release have been observed, mainly due to the interaction of the nanovesicles with high density lipoproteins in blood [Citation15,Citation16]. To minimize side reactions that lead to decreased efficacy of liposomal systems, nanovesicles were biofunctionalized with various compounds, increasing their bioavailability and reducing their absorption by the reticuloendothelial system [Citation12,Citation16].
2.1 Biofunctionalized polymeric liposomal system
Biofunctionalizing the liposomes with polyethylene glycol (PEG) () [Citation15] prevents the interaction between the liposomal nanovesicles and the mononuclear phagocytic system [Citation12,Citation17], obtaining a higher pharmacokinetic response. Li et al. [Citation16] have evaluated the influence of polyethylene glycol (PEG) upon the time that the nanovesicles are retained in the blood. They evaluated LPD complex (liposome-polycation-DNA) [Citation16] which was synthesized using cationic liposome, nucleic acids and protamine [Citation16]. Experimental results highlight that by functionalizing liposomal vesicles with PEG, the pharmacodynamic properties of liposomal systems increase and their inactivation is minimized.
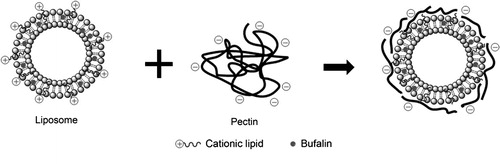
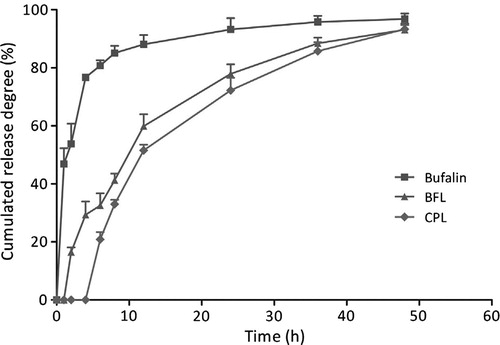
Ying Li and collaborators have investigated the consequences of biofunctionalized controlled release systems composed of liposomal bufalin and pectin [Citation11]. Bufalin [Citation19] represents a chemotherapeutic agent mainly used for colon cancer and gastric cancer [Citation18]. Bufalin administration entails a number of negative effects due to increased toxicity of the active substance and low solubility in water. Thus, it has been administrated in the liposomal form in order to reduce the overconcentration in the body and increase the therapeutical effect. In their study, liposomal bufalin has been functionalized with citrus pectin () – a non-toxic polymer, biodegradable and biocompatible in order to increase the stability of the liposomal complex [Citation11]. Analyses have shown the benefits of biofunctionalization with a biocompatible polymer upon liposomal controlled release systems [Citation11] ().
3 Bupivacaine – structure, mechanism of action, toxicity
3.1 Chemical structure
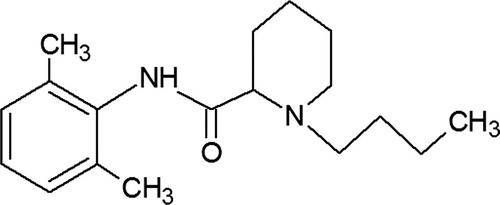
Bupivacaine is a synthesis derivate, with chemical configuration similar to the first local anesthetic ever discovered – cocaine [Citation20–Citation22]. For a local anesthetic agent to be useful, it must have some characteristics: increased potency, sufficient duration of action, low systemic toxicity, low local toxicity, short onset time, high solubility in water, easily sterilizing [Citation20,Citation23,Citation24]. The chemical structure of bupivacaine (see ), highlights different subunits: an aromatic group, an intermediate chain which includes an amide link and an amine group. The liposolubility is due to the aromatic ring, allowing the compound to cross the axonal membrane, essential step in anesthetic action.
Anesthetic liposolubility generates its intrinsic potency and the onset time. Hydrolysis takes place in the amide bond, the degradation is mainly in the liver and the degradation compounds rarely induce allergic reactions [Citation20,Citation25,Citation26]. An increase in molecular volume increases the anesthetic potency to a certain level when it starts to decline. Finally, we can say that the chemical structure influences anesthetic liposolubility, water solubility, pharmacokinetics and eventually block characteristics and clinical effects [Citation27].
3.2 Mechanism of action
Neuronal membrane is characterized by a resting potential generated by electric potential difference (60–90 mV) between the internal and external membrane [Citation28]. The resting membrane is impermeable to Na+ and there is a concentration gradient between intracellular and extracellular environment (14 mmol/L and 142 mmol/L) [Citation29]. The depolarizing process is carried out by electrical charge transfer from outside to inside of the cell membrane until the reversal of the potential at which time the Na+ channels are closed [Citation30]. In the process of repolarization K+ ions move out of the cell to restore the resting membrane potential [Citation21]. Local anesthetics act after diffusion through the nerve sheath and axonal membrane, conversion to ionized form, binding of the ionized molecule to the Na+ channel, blocking the flow of Na+ ions inside the cell, therefore blocking the neuronal membrane depolarization and preventing the spread of the electrical impulse throughout the nerve [Citation31,Citation32]. The main characteristic of the anesthetic agents is the ability to exist in solution, in both unionized and ionized forms [Citation33]. The neutral form is less water soluble, but highly liposoluble which favors crossing the lipidic cell membranes [Citation34–Citation37].
The ionized form is less liposoluble but strong watersoluble, which allows binding to specific receptors on the Na+ channel and determining closing the channels which stops the Na+ flow and prevents nerve fiber depolarization [Citation23,Citation26–Citation28].
3.3 Toxicity of local anesthetics
Systemic toxicity is caused by high concentrations of local anesthetics. They occur accidentally by injection directly into the systemic circulation or by overdose [Citation38]. Most sensitive to the toxic action of local anesthetic agents are central nervous system [Citation39] and cardiovascular system [Citation40,Citation41]. Therefore the symptoms can include isolated muscle contractures, incoherent speech, generalized convulsions, loss of consciousness [Citation42], coma and respiratory depression [Citation43,Citation44].
Cardiovascular changes include tachycardia, slight increase in arterial tension, followed by PR and QRS prolongation [Citation27,Citation41]. AV block or various arrhythmias, decreased cardiac output and accentuated hypotension and ultimately asystole or ventricular fibrillation [Citation34]. Studies have shown that bupivacaine has a higher affinity for cardiac tissue than lidocaine, thus cardiovascular collapse occurs faster. Cai et al. [Citation1] have shown in their study that bupivacaine, tetracaine and etidocaine have a higher tendency to impair the cardiovascular system or depress the central nervous system compared with lidocaine, mepivacaine and prilocaine [Citation1]. The main obstacle in using bupivacaine is pronounced systemic toxicity [Citation45,Citation46].
The toxic concentrations initially cause marked sympathetic stimulation, but later myocardial contractility depression and ventricular arrhythmias carrying the risk of nonresuscitable cardiac arrest [Citation47]. ECG analysis reveals in early stages PR prolongation and QRS widening followed by the appearance of malign arrhythmias [Citation31]. Due to its increased liposolubility and protein binding (heart muscle binding is rapid, but elimination is slow), bupivacaine-induced cardiac arrest is impossible in clinical practice. Pregnant women [Citation48] are susceptible to cardiotoxicity due to the fact that cardiac depression is increased by progesterone [Citation49–Citation51]. In order to limit the toxic consequences of bupivacaine and provide a favorable effect in clinical practice, alternatives have been developed for controlled release of the active substance in order to support the motor and sensory local block for a longer period of time, thus enhancing patient safety.
For this purpose, a number of biomaterials are being studied: lipospheres, microspheres [Citation52], cyclodextrin matrices, lipid–protein–sugar nanoparticles, microcrystals, implantable pellets, implantable membranes and, not least liposomal bupivacaine [Citation53–Citation57].
4 Liposomal bupivacaine
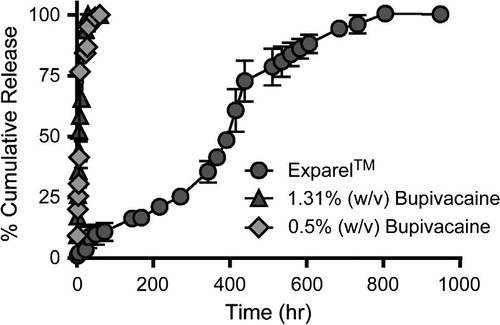
Liposomal bupivacaine has recently been introduced in clinical practice [Citation56,Citation57] and is tested in many clinical studies on healthy volunteers in order to obtain long-lasting pain relief in a single dose administration (DepoFoam bupivacaine, Exparel™) [Citation53]. Liposomal bupivacaine consists of liposomal spheres with diameter between 31.2 μm ± 17.8 [Citation53]. In vitro studies regarding controlled release system compared the Exparel™ complex with bupivacaine hydrochloride 0.5% (w/v) and 1.31% respectively, with an equal amount of active substance. Release kinetics profile was identical in the case of hydrochloride from free bupivacaine with a maximum time release of 48 h, while regarding the Exparel™ complex [Citation53,Citation57], the active substance has been fully released in about 800 h ().
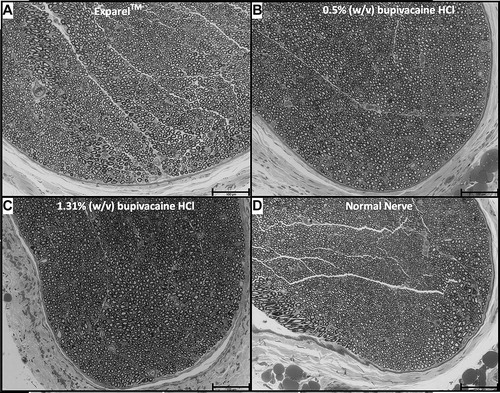
McAlvin et al. [Citation57] studied the effect of liposomal bupivacaine on the sciatic nerve in experimental models, and obtained sensory block for 240 min for the Exparel™ complex and 120 min for bupivacaine hydrochloride 0.5% (w/w) [Citation57]. After histological evaluation, they found that both Exparel complex and bupivacaine hydrochloride produce tissue reaction, the liposomal complex being less aggressive. The degree of mycotoxicity was similar in both pharmaceutical preparations, which was examined 2 weeks after administration. Also, the perineural tissue appears normal, without emphasizing significant changes in axonal density and myelin structure [Citation57] ().
Lonner et al. [Citation58] studied the role of liposomal bupivacaine in pain management after total joint arthroplasty, and observed that liposomal bupivacaine pharmacokinetics and pharmacodynamics are supporting a minimum concentration necessary to maintain the therapeutical effect for 72 h without overconcentrating the active substance in the body [Citation58]. Thus, liposomal bupivacaine has less cardiac toxic effects, without significant differences between Exparel™ and placebo: palpations and extrasystoles (⩽2%) [Citation58], tachycardia (3.9%) [Citation58], bradycardia (1.6%) [Citation58], hypertension and hypotension (⩽2%) [Citation58].
Richard et al. [Citation59] showed no significant hematological, biochemical and biological side effects of Exparel complex in laboratory animals. Histological analysis 15 days after administrating Exparel™ formulation showed evidence of granulomatous inflammation that was resolved without significant tissue damage. The optimum plasma concentration was maintained for about 96 h [Citation59]. Liposomal bupivacaine used in postoperative patients reduces the need for opioids, the hospitalization period and costs by up to 50%, as shown by Cohen [Citation60] and collaborators in clinical trials involving sigmoidectomy, cecectomy, hemicolectomy (right or left) in adult patients [Citation60].
Soberón et al. [Citation61] present the case of a 45 year old woman with digital ischemia (fingers 4 and 5) at the right hand. Axillary block was obtained by injecting 3 mL Exparel 1.3% [Citation61]. They found that the results are superior to the subclavicular block due to a better pain control [Citation61]. After surgery, photoplethysmography showed a normal ulnar artery and disappearance of finger cyanosis, which was due to the vasodilation effect created by liposomal bupivacaine [Citation61–Citation64].
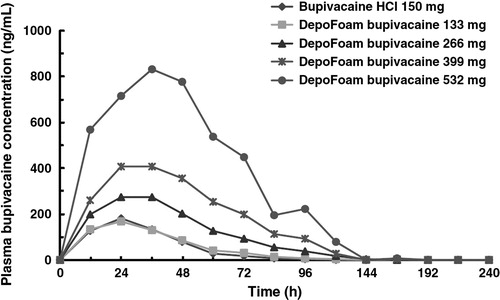
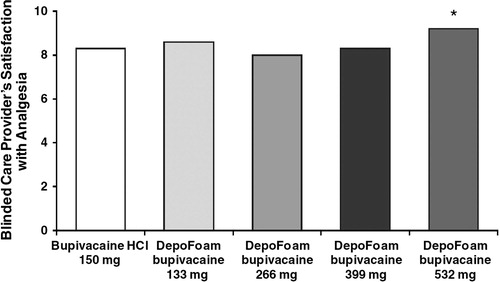
Bramlett et al. [Citation62] in a study on analgesic effect of liposomal bupivacaine in patients after total knee arthroplasty, describe replacing classical continuous femoral blocks [Citation63] with liposomal bupivacaine Exparel, leading to increased patient comfort. They also note that for a proper analgesia up to 96 h 532 mg of Bupivacaine DepoFoam 1.3% in just one administration (Exparel™) is necessary [Citation62] ( and ).
5 Conclusions
The use of liposomal bupivacaine in order to obtain motor and sensory block represents a breakthrough in medical practice. Long-term analgesia in postoperative patients achieved by liposomal bupivacaine controlled release systems represents a great advantage for the safety and patient comfort. Therapeutical action of liposomal bupivacaine sustained up to 96 h after one administration without toxic concentration, well tolerated, without side effects and exhibited a predictable kinetic profile.
6 Conflict of interest
The authors have no conflict of interest to declare.
Notes
Available online 13 January 2015
References
- X.Y.CaiL.M.XiongS.H.YangComparison of toxicity effects of ropivacaine, bupivacaine, and lidocaine on rabbit intervertebral disc cells in vitroSpine J1432014483490
- I.CiucaL.PopZ.PopaL.TamasB.A.GutaP.Matusz187 evaluation of cystic fibrosis liver disease and the relation with risk factors in a Romanian centreJ Cyst Fibros132014S94
- N.M.MekawyS.S.I.BadawyS.SakrRole of postoperative continuous subfascial bupivacaine infusion after posterior cervical laminectomy: randomized control studyEgypt J Anaesth28120128388
- P.SzmukP.OlomukR.B.PopGeneral anesthetics and neurotoxicity in the developing brain: a review of current literatureJ Rom Anesth Terap Int1722010117122
- G.KuthialaG.ChaudharyRopivacaine: a review of its pharmacology and clinical useIndian J Anaesth5522011104110
- M.S.MahmoudA.A.Abd Al AlimA.F.HefniDexamethasone bupivacaine versus bupivacaine for peribulbar block in posterior segment eye surgeryEgypt J Anaesth2942013407411
- I.AcalovschiPostoperative nausea and vomitingCurr Anaesth Crit Care13120023743
- B.Al-NasserLong-acting local anesthetics at home: do we need to worry?J Clin Anesth1932007239240
- R.BaxterK.BramlettE.OnelS.DanielsImpact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studiesClin Ther3532013312320
- D.ZuckerD.MarcusY.BarenholzA.GoldblumLiposome drugs’ loading efficiency: a working model based on loading conditions and drug’s physicochemical propertiesJ Control Release139120097380
- Y.LiH.ZhaoL.R.DuanPreparation, characterization and evaluation of bufalin liposomes coated with citrus pectinColloid Surfaces A Physicochem Eng Asp44420145462
- T.M.AllenP.R.CullisLiposomal drug delivery systems: from concept to clinical applicationsAdv Drug Deliv Rev65120133648
- F.QiuR.MhannaL.ZhangY.DingS.FujitaB.J.NelsonArtificial bacterial flagella functionalized with temperature-sensitive liposomes for controlled releaseSens Actuat B Chem1962014676681
- K.E.UhrichS.M.CannizzaroR.S.LangerK.M.ShakesheffPolymeric systems for controlled drug releaseChem Rev9911199931813198
- K.Y.LeeS.H.YukPolymeric protein delivery systemsProg Polym Sci3272007669697
- S.-D.LiL.HuangNanoparticles evading the reticuloendothelial system: role of the supported bilayerBiochim Biophys Acta178810200922592266
- H.K.KimelbergDifferential distribution of liposome-entrapped [3H]methotrexate and labelled lipids after intravenous injection in a primateBiochim Biophys Acta – Biomembr44841976531550
- Y.LuoA.WangJ.YuanQ.GaoPreparation, characterization and drug release behavior of polyion complex micellesInt J Pharm3741–22009139144
- B.MaZ.Y.XiaoY.J.ChenSynthesis and structure–activity relationships study of cytotoxic bufalin 3-nitrogen-containing-ester derivativesSteroids7852013508512
- X.Y.LiY.ZhaoM.G.SunMultifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain gliomaBiomaterials3521201455915604
- J.B.CelikIntraarticular local anesthetics: primum non nocereKnee Surg Sports Traumatos Arthrosc2020122124
- H.McLureP.RubinReview of local anaesthetic agentsMinerva Anestesiol71320055974
- V.R.ShahR.MackwanaK.VoraSpinal anaesthesia in ambulatory surgery: dose-response characteristics of constant volume bupivacaineIndian J Anaesth5222008191195
- S.LeoneS.Di CianniA.CasatiG.FanelliPharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaineActa Biomed792200892105
- Y.C.I.TongA.D.KayeR.D.UrmanLiposomal bupivacaine and clinical outcomesBest Pract Res Clin Anaesthesiol28120141527
- D.OdedraG.LyonsCurrent anaesthesia & critical care local anaesthetic toxicityCurr Anaesth Crit Care21120105254
- G.L.WeinbergR.RipperP.MurphyLipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heartReg Anesth Pain Med3142006296303
- R.GoyalR.N.ShuklaLocal anesthetic systemic toxicity (LAST) – should we not be concerned?Med J Armed Forces India6842012371375
- A.J.Rodriguez-NavarroM.LagosC.FigueroaPotentiation of local anesthetic activity of neosaxitoxin with bupivacaine or epinephrine: development of a long-acting pain blockerNeurotox Res1642009408415
- D.UptakeMembrane biology effects of local anesthetics on phospholipid topology and dopamine uptake and release in rat brain synaptosomes materials and methods preparation of synaptosomesJ Membrane Biol2181982211218
- E.De PaulaC.M.S.CeredaG.R.TofoliM.Franz-MontanL.F.FracetoD.R.de AraújoDrug delivery systems for local anestheticsRecent Pat Drug Del Formul4120102334
- A.BorgeatG.EkatodramisS.BlumenthalInterscalene brachial plexus anesthesia with ropivacaine 5 mg/mL and bupivacaine 5 mg/mL: effects on electrocardiogramReg Anesth Pain Med2962004557563
- J.SafariZ.ZarnegarAdvanced drug delivery systems: nanotechnology of health design a reviewJ. Saudi Chem Soc1820148599
- F.S.JungermanH.N.P.AlvesM.J.C.CarmonaN.B.ContiA.MalbergierAnesthetic drug abuse by anesthesiologistsRev Bras Anestesiol6232012375386
- H.TsuchiyaT.UenoM.MizogamiK.TakakuraLocal anesthetics structure-dependently interact with anionic phospholipid membranes to modify the fluidityChem Biol Interact183120101924
- J.H.ChoiJ.Y.JangY.K.JoungM.H.KwonK.D.ParkIntracellular delivery and anti-cancer effect of self-assembled heparin-Pluronic nanogels with RNase AJ Control Release14732010420427
- N.G.PortneyM.OzkanNano-oncology: drug delivery, imaging, and sensingAnal Bioanal Chem38432006620630
- R.T.WilderLocal anesthetics for the pediatric patientPediatr Clin North Am4732000545558
- K.ByrneC.EngelbrechtToxicity of local anaesthetic agentsTrends Anaesth Crit Care3120132530
- Z.WangJ.ShenJ.WangLithium attenuates bupivacaine-induced neurotoxicity in vitro through phosphatidylinositol-3-kinase/threonine-serine protein kinase B- and extracellular signal-regulated kinase-dependent mechanismsNeuroscience2062012190200
- M.K.ClarkLipid emulsion as rescue for local anesthetic-related cardiotoxicityJ Perianesth Nurs2322008111117
- K.T.RiutortC.B.RobardsS.R.ClendenenS.J.BrullR.A.GreengrassContinuous peripheral nerve blockade may contribute to early diagnosis of postoperative myocardial infarction in high risk patientsJ Rom Anest Terap Int18120114952
- L.FerrariV.CrestanG.SabattiniF.VincoS.FontanaA.GozziBrain penetration of local anaesthetics in the rat: implications for experimental neuroscienceJ Neurosci Methods18622010143149
- R.PaderaE.BellasJ.Y.TseD.HaoD.S.KohaneLocal myotoxicity from sustained release of bupivacaine from microparticlesAnesthesiology10852008921928
- X.ShenF.WangS.XuIs cardiolipin the target of local anesthetic cardiotoxicity?Rev Bras Anestesiol6042010445454
- C.KangS.-C.ShinDevelopment of prilocaine gels for enhanced local anesthetic actionArch Pharm Res357201211971204
- H.TsuchiyaT.UenoM.MizogamiStereostructure-based differences in the interactions of cardiotoxic local anesthetics with cholesterol-containing biomimetic membranesBioorg Med Chem1911201134103415
- K.J.S.AnandC.C.JohnstonT.F.OberlanderA.TaddioV.T.LehrG.WalcoAnalgesia and local anesthesia during invasive procedures in the neonateClin Ther2762005844876
- D.KothariJ.BindalImpact of obstetric analgesia (regional vs parenteral) on progress and outcome of labour: a reviewJ Rom Anest Terap Int18120113440
- L.ColR.GoyalC.R.N.ShuklaReview article local anesthetic systemic toxicity (LAST) and should we not be concerned?Med J Armed Forces India8201259
- H.TsuchiyaM.MizogamiStereospecific cardiotoxicity evaluation of local anesthetics using their discriminable interactions with biomimetic chiral membranesToxicol Lett2212013S95S96
- R.A.GreengrassM.W.IsenhowerK.RospigliosiS.J.BrullContinuous peripheral nerve catheters and regional analgesia in pregnancy – a brief review of safetyJ Rom Anest Terap Int2022013121124
- R.M.De OliveiraP.P.TanakaS.B.TenorioAssessing the use of 50% enantiomeric excess bupivacaine-loaded microspheres after sciatic nerve block in ratsRev Bras Anestesiol6162011736747
- J.B.McAlvinR.F.PaderaS.A.ShankarappaMultivesicular liposomal bupivacaine at the sciatic nerveBiomaterials3515201445574564
- F.MaestrelliM.L.González-Rodrígueza M.RabascoC.GhelardiniP.MuraNew, “drug-in cyclodextrin-in deformable liposomes” formulations to improve the therapeutic efficacy of local anaestheticsInt J Pharm3951–22010222231
- N.SermkaewW.KetjindaP.BoonmeN.PhadoongsombutR.WiwattanapatapeeLiquid and solid self-microemulsifying drug delivery systems for improving the oral bioavailability of andrographolide from a crude extract of Andrographis paniculataEur J Pharm Sci503–42013459466
- J.B.McalvinR.F.PaderaS.A.ShankarappaBiomaterials Multivesicular liposomal bupivacaine at the sciatic nerveBiomaterials3515201445574564
- J.LonnerRole of liposomal bupivacaine in pain management after total joint arthroplastyJ Surg Orthop Adv230120143741
- B.M.RichardP.NewtonL.R.OttThe safety of EXPAREL ® (bupivacaine liposome injectable suspension) administered by peripheral nerve block in rabbits and dogsJ Drug Del20122012110
- S.M.CohenExtended pain relief trial utilizing infiltration of Exparel(®), a long-acting multivesicular liposome formulation of bupivacaine: a Phase IV health economic trial in adult patients undergoing open colectomyJ Pain Res52012567572
- J.R.SoberónS.F.DuncanW.C.SternberghCase report treatment of digital ischemia with liposomal bupivacaineCase Rep Anesth2014201414
- K.BramlettE.OnelE.R.ViscusiK.JonesA randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam Bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arhroplastyKnee1952012530536
- A.V.CheeJ.RenB.A.LenartE.-Y.ChenY.ZhangH.S.AnCytotoxicity of local anesthetics and nonionic contrast agents on bovine intervertebral disc cells cultured in a three-dimensional culture systemSpine J1432014491498
- M.AntoniJ.Y.JennyE.NollPostoperative pain control by intra-articular local anesthesia versus femoral nerve block following total knee arthroplasty: impact on dischargeOrthop Traumatol Surg Res10032014313316