 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Hyperglycemia is common among critically ill patients and is associated with increased morbidity and mortality and there is no clear answer to the question: which to apply tight or conventional glycemic control?
Objective
Evaluation and comparison of the effects of tight versus conventional glycemic control on critically ill patients in our surgical intensive care unit (ICU).
Design
Prospective randomized controlled trial.
Methods
120 Patients were divided into two groups: group (I) received intensive insulin therapy targeting blood glucose level between 80 and 110 mg/dl, who referred to as intensive treatment group, and group (II) received conventional insulin therapy targeting blood glucose level between 150 and 200 mg/dl, and referred to as conventional treatment group.
Results
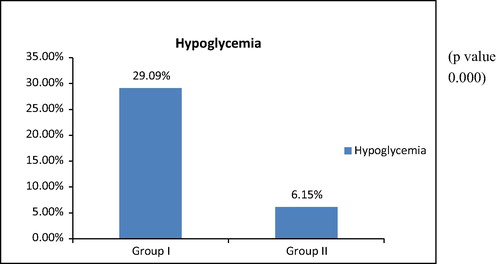
120 Patients were enrolled in the study, the incidence of hypoglycemia (blood glucose <70 mg/dl) was 29.09% in group I who received intensive insulin therapy versus 6.15% in group II who received conventional insulin therapy (p value 0.000) with no demonstrable complications, regarding mortality rate, impairment of Liver function tests, change in total leukocytic count, the need for red blood cell transfusion, ICU stay and Total hospital stay and we reported no statistical significant difference between the two groups.
Conclusion
Tight glycemic control for critically ill patients in ICU in poor resources countries showed increased incidence of hypoglycemia with no significant benefits when compared with conventional glycemic control.
Keywords:
Introduction
Hyperglycemia is common among critically ill patients and is associated with increased morbidity and mortality, and in the past decades there was strong recommendation for tight glycemic control [Citation1–Citation3] as Van den Berghe et al. [Citation4] reported a dramatic 42% relative reduction in mortality in the surgical Intensive Care Unit (ICU) when blood glucose was normalized to 80–110 milligram per deciliter (mg/dl) by means of insulin infusion in a prospective, randomized fashion. However this strategy was associated with increased risk of hypoglycemia [Citation5–Citation6]. Few years later, the same authors demonstrated no mortality benefit from intensive glucose control in their medical ICU, except in a subgroup requiring critical care for 3 or more days [Citation7].
able 2 Systolic blood pressure in group I (55 patients) and group II (65 patients). Data are represented in terms of mean ± standard deviation.
able 3 Heart rate in group I (55 patients) and group II (65 patients). Data are represented in terms of mean ± standard deviation.
able 4 Central venous pressure in group I (55 patients) and group II (65 patients). Data are represented in terms of mean and standard deviation.
able 5 Creatinine in group I (55 patients) and group II (65 patients). Data are presented in terms of mean ± standard deviation.
able 6 Liver enzymes in group I (55 patients) and group II (65 patients). Data are presented in terms of mean ± standard deviation.
able 7 Hemoglobin (Hb) in group I (55 patients) and group II (65 patients) in gm/dl. Data are presented in terms of mean ± standard deviation.
able 8 Total leukocytic count (TLC) in group I (55 patients) and group II (65 patients). Data are presented in terms of mean ± standard deviation.
The Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial compared Intensive and conventional glycemic control in a randomized, unblinded fashion in 6104 patients in the ICU and demonstrated that intensive insulin therapy (target 81–108 mg/dL) in critically ill patients was associated with increased 90-day mortality when compared with conventional treatment (target 180 mg/dL) [Citation8].
So into this controversy the question was If intensive insulin therapy targets blood glucose level (80–110 mg/dL) can be proven effective in optimal conditions, how to make that benefit available to millions of critically ill patients in both developed and poor resources countries around the world.
There is no clear answer to the complex problem of glycemic control in critically ill adults; at present, targeting tight glycemic control cannot be said to be either right or wrong.
Aim of the work
Our objective was to evaluate and compare the effects of tight glycemic versus conventional glycemic control on critically ill patients in our surgical intensive care unit (ICU) regarding mortality, incidence of hypoglycemia, ICU length of stay, total hospital stay and occurrence of complications as sepsis and organ dysfunction e.g.; acute respiratory distress syndrome (ARDS), hemodynamic instability, renal and hepatic dysfunction and need for red cell transfusion.
Methods
.1 Study population
After getting the approval from Ethics and Research Committee of Anesthesia Department, Faculty of Medicine, Cairo University and obtaining written informed consents, all postoperative critically ill hyperglycemic patients between 20 and 70 years admitted to surgical intensive care unit during the period 2010–2012 were included in the study excluding patients with sepsis, hemodynamic instability, ARDS, renal dysfunction (creatinine above 2 mmol/l) and chronic hepatic dysfunction.
.2 Sample size calculation
We used standard methods to calculate sample sizes for a trial with 80% power to detect a treatment effect and 95% confidence level. The sample size calculated to detect a confidence interval of 0.5–5 in the percentage incidence of hypoglycemia as a complication of the glycemic control protocols was 102. So we included 120 patients in the different study groups.
.3 Study design
On admission to the intensive care unit, patients were randomly allocated into two groups using a closed envelope group (I) who received intensive insulin therapy to achieve blood glucose level between 80 and 110 mg/dl, and referred to as intensive treatment group, and the other group (II) received conventional insulin therapy targeting blood glucose level between 150 and 200 mg/dl and this group referred to as conventional treatment group.
If patients were on insulin therapy before ICU admission so hyperglycemic critically ill patients were classified according to total insulin dose in the preceding 24 h before admission into insulin-sensitive (who received <50 units insulin/day and those patients who were not on insulin therapy), usual (received 50–100 units insulin/day), or insulin-resistant (who received >100 units insulin/day) categories. After enrollment, venous blood samples were sent to the laboratory to determine basal blood glucose level and correlate the result with that determined with the glucometer, and this is to know the error factor between the two results as glucometer was used during rest of the day. Capillary blood obtained via finger stick was checked every hour until 4 successive values within the target range: (80–110 mg/dl) in intensive treatment group and (150–200 mg/dl) in conventional treatment group.
Once the target range was achieved, blood glucose values were checked every 2 h.
Management of hypoglycemia: if the blood glucose level was less than 70 mg/dl, the insulin infusion was stopped, and the patient was given 50 ml of 25% dextrose in water as a slow intravenous infusion over 5 min and the blood glucose level was checked every 15 min for 3 times.
For patients received total parenteral nutrition, insulin was not added to their total parenteral feeding except when daily insulin requirements exceeded 50 units, in which case two-thirds of the previous day’s total insulin dose was added to the next feeding.
Intensive glucose control: target blood glucose level 80–110 mg/dl. No insulin infusion was started if the initial blood glucose level was 110 mg/dl or less. If the initial blood glucose level was greater than 110 mg/dl but less than 500 mg/dl, then an insulin infusion was started at a rate (blood glucose level in mg/dl) × 0.01 units/h. If the initial blood glucose level was 500 mg/dl or greater, then an insulin infusion was started at 6 units/h.
For blood glucose levels between 201 and 250 mg/dl, the insulin infusion was increased by 3–4 units/h and an intravenous bolus of regular insulin was given 2–3 units for an insulin sensitive subject, 4–5 units for a usual subject, and 6–8 units for an insulin-resistant subject. For blood glucose levels between 141 and 200 mg/dl, the insulin infusion was increased by 1–2 units/h and intravenous bolus of regular insulin was given 2 units for an insulin sensitive subject, 3 units for a usual subject, and 6 units for an insulin resistant subject. For blood glucose levels between 111 and 140 mg/dl the insulin infusion was increased by 0.5–1 unit/h. For blood glucose levels between 80 and 110 mg/dl the insulin infusion will be continued at the same rate. For blood glucose levels between 70 and 79 mg/dl, the insulin infusion was decreased 1–2 units/h.
For blood glucose levels less than 70 mg/dl, hypoglycemia management was initiated as described above. When blood glucose level exceeded 95 mg/dl, the insulin infusion was resumed again.
Conventional glucose control: target blood glucose level 150–200 mg/dl. No insulin infusion was started if the initial blood glucose level was 200 mg/dl or less. If the initial blood glucose level was greater than 200 mg/dl, but less than 500 mg/dl, then an insulin infusion was started at a rate (blood glucose level in mg/dl) × 0.01 units/h. If the initial blood glucose level was greater than 500 mg/dl, then an insulin infusion was started at 6 units/h.
For blood glucose levels between 251 and 300 mg/dl, the insulin infusion was increased by 1–2 units/h and an intravenous bolus of regular insulin was given 2 units for an insulin sensitive-subject, 3 units for a usual subject, and 6 units for an insulin-resistant subject. For blood glucose levels between 201 and 250 mg/dl, the insulin infusion will be increased by 0.5–1 unit/h. For blood glucose levels between 150 and 200 mg/dl the insulin infusion was continued at the same rate. For blood glucose levels between 120 and 149 mg/dl, the insulin infusion was decreased by 1–2 units/h. For blood glucose levels between 70 and 119 mg/dl, the insulin infusion was decreased 2–3 units/h.
For blood glucose levels less than 70 mg/dl, hypoglycemia management as described above will be initiated. When the blood glucose level exceeded 190 mg/dl, the insulin infusion was resumed again.
.4 Monitoring and measurement
On ICU admission a complete history and physical examination were recorded for each patient enrolled in the study.
| #x2022; | APACHE II (“Acute Physiology and Chronic Health Evaluation II”) score [Citation9] was calculated. | ||||
| #x2022; | Blood samples were obtained by finger lancet or from an arterial catheter. Blood glucose level was measured at the bed side with (One Call Plus) glucometer and (One Call Plus) test strips. | ||||
| #x2022; | HbA1C (Glycosylated hemoglobin). | ||||
| #x2022; | Blood pressure, heart rate and central venous pressure were monitored to assess hemodynamic stability. | ||||
| #x2022; | Blood gases to assess respiratory functions. | ||||
| #x2022; | Liver and kidney functions (ALT, AST, urea and creatinine). | ||||
| #x2022; | Complete Blood Count (CBC) to assess bone marrow function. | ||||
.5 Data collection
Age, sex, body weight, APACHE II score, and associated comorbidities were recorded on admission for each patient. Blood glucose level will be recorded on admission; subsequently, every 4 h, daily at 8 am and daily maximal and minimal blood glucose levels were determined. Mortality rate, number of organ failure, ICU stay and total hospital stay were recorded for every patient.
Systolic blood pressure, heart rate, central venous pressure, hemoglobin, total leukocytic count, creatinine, Alanine Transaminase and Aspartate Transaminase enzymes were recorded during first five days of starting glycemic control after admission in ICU.
.6 Statistical analysis
Data will be analyzed using SPSS 13 program and microsoft excel 2007. Numerical data will be presented as means, modes, ranges of minimum and maximum as well as the standard deviation of the mean from the study population. Also, ordinal and categorical data will be presented as numbers and percent of total. Groups will be compared using one way and two way analysis of variance and Kruskal Wallace analysis of variance as appropriate to the data to be analyzed.
Results
There was no statistical significant change between the two groups regarding systolic blood pressure during the first five days of starting glycemic control.
There was no statistical significant change in the heart rate between the two groups during the first five days of starting glycemic control.
There is no statistical significant difference in the central venous pressure between the two groups during the first five days of starting glycemic control.
Patients needed inotropic support in group I represented (14.5%) while in group II were (6.1%).
Creatinine level showed no statistical significant change between the two groups during the first five days after starting glycemic control.There was no statistical significant difference in hemoglobin level between the two groups during the first five days of starting glycemic control.
Total leukocytic count showed no statistical significant difference between the two groups during the first five days after starting glycemic control.
There was no statistical significant difference between group I and group II regarding ICU and total hospital stay.
14.5% of patients in group I and 9% in group II were on steroid therapy, almost all patients of group I needed insulin therapy while only 44.6% of group II needed insulin therapy (see. and –).
able 1 Demographic data of IIT group (55 patients, group I) and conventional group (65 patients, group II). Data are represented as mean ± standard deviation (SD) and percentage.
able 9 ICU and total hospital stay in group I (55 patients) and group II (65 patients) in days. Data are presented in terms of mean ± standard deviation.
Discussion
The current study demonstrated that patients who received intensive insulin therapy showed higher incidence of hypoglycemia and mortality rates with no obvious difference in complications of the cardio-respiratory system, liver and kidney functions when they were compared with conventional glycemic control group (group II).
In this study incidence of hypoglycemia (blood glucose <70 mg/dl) was 29.09% in group I and 6.15% in group II with (p value 0.00) with no demonstrable complications.
These results were in line with NICE SUGAR trial which is conducted in a randomized, unblinded fashion over 6104 ICU patients, in 41 hospitals in Australia, New Zealand, and Canada and at the Mayo Clinic in the United States and reported that 6.8% of patients in the intensive-control group experienced severe hypoglycemia (blood glucose level 40 mg/dl) versus 0.5% in the conventional-control group (P < 0.001) [Citation8].
Two multicenter studies [Citation5,Citation6] reported unacceptably high rates of hypoglycemia, and one trial was prematurely terminated for this reason [Citation6].
The higher incidence of hypoglycemia in our study may be due to the higher reference value (70 mg/dl) than that is considered in other mentioned studies and also decreased ratio of nurses per ICU bed.
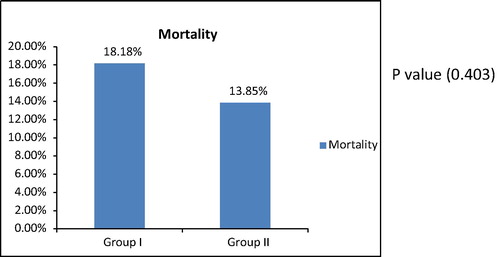
Our study reported insignificant increase in mortality rates in group I (18.18%) versus (13.85%) in group II, with a P value 0.40.
NICE SUGAR study reported that the total mortality in the intensive-control group was (27.5%) and in the conventional-control group was (24.9%) with P value 0.02 [Citation8].
The difference of Values of mortality rates may be due the long term follow-up of patients (90 days) in NICE SUGAR study, while it is limited to the period of hospital stay in the current study.
On the contrary Van den Berghe et al. [Citation4] conducted a study on postoperative mechanically ventilated patients after cardiac surgery, with a total number of 1548 patients enrolled and reported 4.6% mortality rate in intensive treatment group, as compared with 8.0% in the conventional-treatment group, representing an apparent risk reduction of 42% (95% confidence interval, 22–62%). This may be due to the quality of patients and the special type of care that those patients may be received.
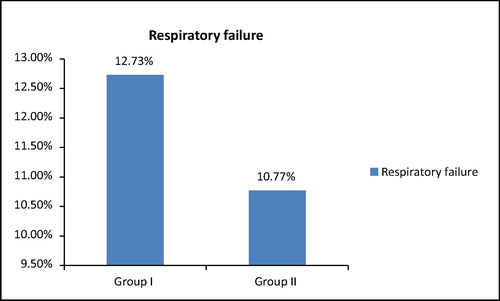
In the current study 14.5% patients of group I versus 6.1% in group II needed inotropic or vasopressor support and there was insignificant difference between the two groups regarding systolic blood pressure and the percentage of patients who experienced respiratory failure during their course in ICU (12.73% in group I versus 10.77% in group II).
On the contrary Greet Van den Berghe et al. demonstrated that 75% of patients on tight glycemic control needed inotropic or vasopressor support versus 74.8% in the conventional treatment group.
Regarding respiratory complications Van den Berghe et al. compared duration of mechanical ventilation between the two groups and reported that there was statistical significant decrease in number of patients on tight glycemic control, while in the NICE SUGAR study there was statistical insignificant difference between the two groups [Citation8].
The current study showed statistical insignificant increase in serum creatinine between the two groups which is in line with NICE SUGAR study that demonstrated that there was no significant difference between the two treatment groups regarding renal replacement therapy (P = 0.39).
Renda Soylemenz et al.’s meta analysis including Twenty-nine randomized controlled trials with total number of 8432 patients, reported that Tight glucose control was not associated with significant risk reduction for new need for dialysis (11.2% versus 12.1% in IIT and conventional groups respectively) [Citation10].
In contrast to our study, Van den Berghe et al. reported that 12.3% of the conventional control group showed statistical significant increase in creatinine level (creatinine >2.5 mg/dl) versus 9% in IIT group.
Regarding impairment of Liver function tests, change in total leukocytic count, the need for red blood cell transfusion, ICU stay and Total hospital stay we reported no statistical significant difference between the two groups; however, Greet Van Den Berghe et al. reported that patients who experienced deterioration in liver functions in IIT group represented 22.4% versus 26.7% in conventional group with P value 0.04, and reported that incidence of septicemia in IIT group was 4.2% while in conventional group was 7.8% with P value 0.003 [Citation4].
However Renda Soylemenz et al.’s meta analysis reported significantly decreased risk of septicemia (10.9% versus 13.4% in IIT and conventional groups respectively) [Citation10].
The NICE SUGAR study and Van den Berghe et al. reported no statistical significant difference regarding ICU stay and Total hospital stay between the two groups [Citation8].
In this study 100% of patients of group I needed insulin therapy while only 44.6% of group II needed insulin therapy. Van den Berghe et al., reported that all patients of intensive insulin group were treated with insulin and only 39% of the patients treated with the conventional approach received insulin [Citation4].
In the NICE-SUGAR trial, 69% of subjects in the conventional treatment group received insulin and, 97% of subjects in the intensive treatment group received insulin [Citation8].
Limitations
Nurse to patient ratio was 2:1 in most cases but sometimes was 3:1 so it was mandatory to omit these patients from intensive treatment group to avoid higher incidence of hypoglycemia (5 patients).
Length of stay in ICU is higher than the expected values due to the delay in discharge in some cases because of the miscommunication between ICU and wards.
We have no specific isolation system in our ICU, so the incidence of infection was higher than expected.
Conclusion
Tight glycemic control for critically ill patients in ICU in poor resources countries showed increased incidence of mortality and hypoglycemia with no significant benefit when compared with conventional glycemic control.
Conflict of interest
Professor Dr. Waleed Hamimy is an Author and is the chief editor of the Egyptian Journal of Anesthesia.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- R.P.DellingerJ.M.CarletH.MasurSurviving Sepsis Campaign guidelines for management of severe sepsis and septic shockCrit Care Med322004858873
- R.P.DellingerM.M.LevyJ.M.CarletInternational Surviving Sepsis Campaign Guidelines Committee Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shockCrit Care Med3612008296327 [Published correction appears inCrit Care Med. 2008;36(4):1394-1396]
- A.J.GarberE.S.MoghissiE.D.BransomeJrAmerican College of Endocrinology position statement on inpatient diabetes and metabolic controlEndocr Pract1020047782
- G.van den BergheP.WoutersF.WeekersIntensive insulin therapy in critically ill patientsN Engl J Med345200113591367
- P.DevosJ.C.PreiserC.MelotImpact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycaemia: final results of the Glucontrol studyIntensive Care Med33Suppl 22007S189
- F.M.BrunkhorstC.EngelF.BloosIntensive insulin therapy and pentastarch resuscitation in severe sepsisN Engl J Med3582008125139
- G.Van den BergheA.WilmerG.HermansIntensive insulin therapy in the medical ICUN Engl J Med3542006449461
- The NICE-SUGAR Study InvestigatorsIntensive versus conventional glucose control in critically ill patientsN Engl J Med360200912831297
- W.A.KnausE.A.DraperD.P.WagnerJ.E.ZimmermanAPACHE II: a severity of disease classification systemCritical Care Med13101985818829
- R.S.WienerD.C.WienerR.J.LarsonBenefits and risks of tight glucose control in critically ill adults: a meta-analysisJAMA3002008933944
