Abstract
Background
Extra-corporeal shock wave lithotripsy (ESWL) is a painful procedure. Sufficient analgesia is mandatory to achieve good treatment results, as well as patient compliance and comfort. Dexmedetomidine, owing to its sedative and its analgesic effects, may be suitable for conscious sedation during ESWL. The aim of this study was to evaluate the use of dexmedetomidine compared with propofol for its safety and efficacy during ESWL.
Patients and methods
Fifty-two patients were randomly divided into 2 groups that received either dexmedetomidine or propofol for elective ESWL. A dose of 1.5 μg/kg of fentanyl was given intravenously (IV) to all patients 10 min before the ESWL procedure. In the dexmedetomidine group, patient received an initial loading dose of 1 μg/kg of dexmedetomidine infused IV over 10 min, followed by an infusion rate of 0.3 μg/kg/h. In the propofol group, the initial loading dose of 1 mg/kg of propofol was infused IV over 10 min, followed by an infusion rate of 3 mg/kg/h. The Observer’s Assessment of Alertness/Sedation (OOA/S) scores, visual analog scale (VAS), and hemodynamic and respiratory variables were recorded regularly at 5-min interval during ESWL. Hospital discharge time was determined according to Kortilla’s criteria for outpatient surgeries.
Results
The OOA/S scores in the dexmedetomidine group at the 25- to 45-min assessments were significantly lower than those seen in the propofol group (P < 0.05). The VAS scores for the dexmedetomidine group were significantly lower than those in the propofol group, but only at the 30- to 45-min assessments (P < 0.05). During sedation, the respiratory rate with dexmedetomidine was significantly slower (P < 0.05). Other clinical variables, adverse effects, and hospital discharge times were comparable in both groups (P > 0.05).
Conclusion
Dexmedetomidine with fentanyl can be used safely and effectively, and it may be a valuable alternative to propofol with fentanyl for conscious sedation during ESWL.
Introduction
Even with new-generation lithotripters, extra-corporeal shock wave lithotripsy (ESWL) is a painful procedure, and sufficient analgesia is mandatory to achieve good treatment results, patient compliance and comfort [Citation1]. A combination of a sedative hypnotic and an opioid analgesic is commonly used to produce patient analgesia and sedation [Citation2,Citation3]. Fentanyl (Fentanyl–Hameln, 0.1 mg/2 ml) is a potent synthetic narcotic, has a rapid onset and a short duration of action. It is a strong agonist at the μ-opioid receptors and provides an acceptable analgesia condition during ESWL but it has a marked respiratory depressive effect [Citation4]. Propofol (Propofol-Lipuro, 10 mg/ml) is a frequently used sedative hypnotic with minimal analgesic properties, but it may cause respiratory depression; an effect that may be potentiated by the presence of opioids [Citation5,Citation6]. The combination of fentanyl and propofol has shown to be even more potent analgesic in ESWL with mitigating side effects such as respiratory depression, decreasing oxygen saturation, nausea, vomiting, drowsiness, and hypersensitivity reactions [Citation7,Citation8]. Dexmedetomidine (Precedex, 100 μg/ml) is a highly selective α-2 adrenergic receptor agonist that has both analgesic and sedative properties, with minimal effect on ventilation, and thus may be a valuable drug for use during outpatient procedures that cause minimal to mild pain, such as ESWL [Citation9].
This study was designed to compare the sedative, analgesic, hemodynamic, and respiratory effects of dexmedetomidine and propofol in combination with fentanyl during ESWL, as well as the time elapsed to hospital discharge.
Patient and methods
This prospective, randomized, double-blinded comparative clinical study was performed in the Sohag and Qena University Hospitals of the Sohag and the South Valley Universities, Egypt respectively, from June 2014 to December 2014. After approval from the institutional ethical committee of both university hospitals, written informed consent was obtained from each patient. The study sample consists of 52 patients (age range: 20–60 years).
The inclusion criteria were as follows: Patients with an American Society of Anesthesiologists (ASA) physical status of I or II, and scheduled for ESWL to treat a single renal (pelvic or upper calyceal) stone with 700–900 Hounsfield Unit (HU) and without pain.
Exclusion criteria included the following: Age younger than 20 years, a history of drug or alcohol abuse, an allergy to any of the study medications, a second- or third-degree heart block, chronic use of α2-agonist drugs, and current psychiatric or respiratory problems. Patients with urinary tract infections or obstruction, cysteine stones, coagulopathies, skeletal deformities, pregnant women and body weights more than 50% of the ideal body weight were also excluded.
Patients were randomly assigned to receive either dexmedetomidine with fentanyl or propofol with fentanyl. Randomization and enrollment were done using sequentially numbered closed envelopes. All patient assessments were performed by a physician (A.A. Tarik) blinded to the sedation analgesic technique used during the lithotripsy procedure. To reduce the selection and the pre-test biases, an anesthetist (E.I. Darweesh) prepared the study drugs and wrapped the syringe pumps and tubing with opaque covering.
We performed routine preoperative evaluations and investigations. Upon arrival in the ESWL unit, patients were allowed to position themselves on lithotripter table, and baseline measurements of mean arterial blood pressure (MAP), heart rate (HR), respiratory rate (RR), and room air oxygen saturation (SpO2) were obtained and electrocardiographic (ECG) leads were applied for continuous ECG monitoring using a Life Scope monitor (BSM – 2353; Nihon Kohden – Japan). Supplemental O2 (4 L/min) was administrated using an oxygen face mask and, after placement of the intravenous (IV) cannula, 1.5 μg/kg of fentanyl was given to all patients 10 min before the start of ESWL. Patients were randomly divided into 2 groups: patients in the dexmedetomidine group (D group) received a loading dose of dexmedetomidine at 1 μg/kg infused IV over 10 min, followed by a maintenance infusion of 0.3 μg/kg/h, and patients in the propofol group (P group) received a loading dose of propofol at 1 mg/kg infused IV over 10 min, followed by a maintenance infusion of 3 mg/kg/h. The maintenance infusion rate (by syringe pump, B. Braun, Melsungen AG – Germany) of either dexmedetomidine or propofol was adjusted to produce a state of moderate sedation/analgesia formerly called conscious sedation [Citation10].
The ESWL procedure was started at the end of the IV infusion of the loading dose of dexmedetomidine or propofol. All patients received 3500–4000 shocks (60–80 per min) at 18 kV using Dornier equipment. Two to 3 min before the end of the procedure, the drug infusions were discontinued. At the end of the ESWL procedure, patients were transferred to the recovery room. Discharge from the hospital was based on Kortilla’s discharge criteria for outpatient procedures [Citation11].
Data collection and measurements
Baseline measurements of HR, non-invasive MAP, RR, and SpO2 were obtained just prior to the start of the study drug infusions. The sedation level was evaluated using the modified Observer’s Assessment of Alertness/Sedation scale (OAA/S), in which 5, indicates response rapidly to name spoken in normal tone (awake and alert); 4, lethargic response to name spoken in normal tone; 3, response only after name is spoken repeatedly or loudly; 2, response only after mild stimulation or shaking; and 1, no response to mild stimulation or shaking [Citation12].
The OAA/S scores and hemodynamic (HR and MAP) and respiratory (RR and SpO2) variables were recorded every 5 min after the baseline measurement until the termination of the ESWL procedure.
Pain intensity was evaluated on a 100-mm visual analog scale (VAS) where 0 indicated no pain, and 100, severe pain. The VAS scores were recorded every 5 min from the start to the end of the ESWL procedure.
During the procedure, adverse effects caused by excessive sedation and analgesia, such as hypotension (MAP: <90 mmHg), bradycardia (HR: <50 beats/min), or oxygen saturation (SpO2: <92%) were recorded and treated. For hypotension and bradycardia we used 0.9% saline infusion, 5 mg of ephedrine and 0.5 mg atropine; for oxygen saturation issues we used an increased flow of O2 supplementation.
We recorded dose increases due to inadequate sedation and analgesia in either group, stone disintegration as well as other adverse effects, such as nausea, vomiting, and dry mouth. After completion of the ESWL procedure, patients were transported to the recovery room, where they were observed until the time they were judged for discharge, according to Kortilla’s criteria for outpatient procedures [Citation11].
Immediately before discharge from the recovery room, patients were asked to rate their overall pain experience (0, no pain; 1, mild pain; 2, moderate pain; or 3, severe pain) and the degree of their overall satisfaction with the control of their pain (0, inadequate; 1, fair; 2, good; or 3, excellent). We recorded the need for analgesic, antiemetic, and/or sedative medications in the recovery room. Post ESWL follow up was done after 2 weeks using plain X-ray urinary tract and abdominal ultrasonography.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 16.0 (IBM, Chicago, Ill). The sample size was based on the primary outcome measurement of improved analgesia. An average difference of 15 mm on a 100-mm VAS was considered clinically relevant [Citation13]. Based on the results of previous studies done by Kaygusuz et al. [Citation13] it was expected that 95% of the VAS scores would range between 0 and 80, resulting in a mean ± SD of 47 ± 17.7 mm. A sample size of 52 patients (26 in each group) was calculated to be necessary to detect a 15-mm change in VAS scores, with a power of 80% and an α error of 5%. Data are presented as mean ± SD, using number or percentage as appropriate. The proportions of male/female, ASA physical status, nausea and vomiting, overall pain experience scores of 2 and 3, patient satisfaction scores, and time to hospital discharge of the study groups were compared with a χ2 test. The RR, SpO2, HR, MAP, OAA/S and VAS were compared using repeated-measures analysis of variance (ANOVA), with post hoc Tukey’s test. Differences were considered to be significant when the P value was <0.05.
Results
The 2 groups were comparable with respect to demographic data, stone size, ESWL procedure time, and dose increase due to inadequate sedation and analgesia ().
able 1 Demographic data, stone size, ESWL procedure time, and dose increase due to inadequate sedation and analgesia of the study groups.
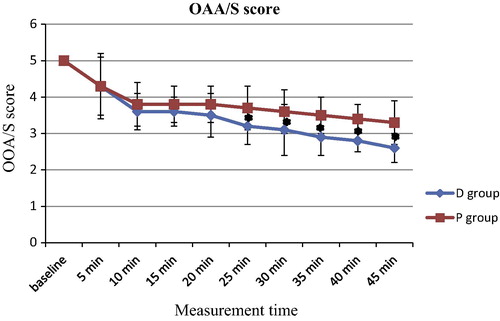
The OOA/S scores at 5–20 min were comparable in both groups (P > 0.05). The OOA/S scores seen in the D group at 25–45 min were significantly lower than those in the P group (P < 0.05). In both groups, the OOA/S scores at 5–45 min were significantly lower than those at the baseline (P < 0.05) ().
The VAS scores at 10 to −25 min were comparable in both groups (P > 0.05), but the VAS scores at 30 to −45 min in the D group were significantly lower than those in the P group (P < 0.05) ().

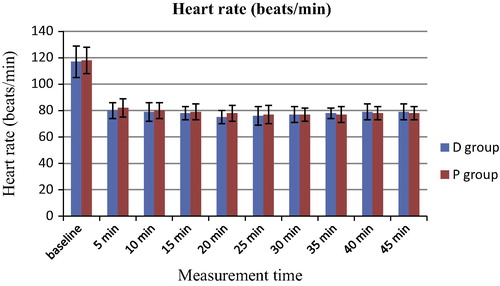
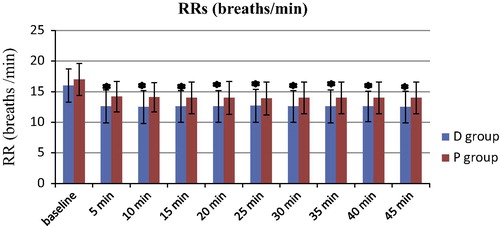
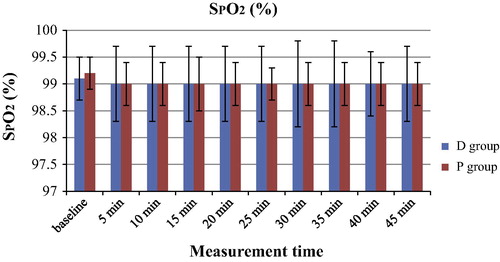
The hemodynamic variables of MAP and HR are shown in and respectively. The MAP values were significantly lower at 5 to −45 min when compared with those at baseline in both groups (P < 0.05). There were no significant differences in the MAP values between the 2 groups (P > 0.05). Similarly, the HR values were significantly lower at 5 to −45 min when compared with those at baseline in both groups (P < 0.05). There were no significant differences in the HR values between the 2 groups (P > 0.05). The RR values of the D group were significantly lower than those in the P group during the sedation period (5–45 min) (P < 0.05) and the RR values during sedation (5–45 min) were significantly lower than those at baseline in both groups (P < 0.05) (). The SpO2 values were similar in both groups (P > 0.05) ().

Adverse effects, such as dry mouth, nausea and vomiting, and patient overall pain experience scores of 2 and 3 were comparable between the 2 study groups (). With regard to patient satisfaction with the sedation method, both methods were assumed to be good or excellent in more than 80% of the patients (). There were no cases of hypotension, bradycardia, or respiratory depression (RR: <8) reported for any patient in the 2 study groups. Stones were completely disintegrated in both groups. Further, no patient required general or local anesthesia to complete the ESWL procedure, nor was the procedure prematurely terminated due to inadequate sedation or analgesia.
able 2 Adverse effects and overall pain experience score of the study groups.
able 3 Patient satisfaction score of the study groups.
The time to hospital discharge, considered as the time from the end of the ESWL procedure, was 87.1 ± 27.5 min and 85 ± 21.4 min in the D group and P group, respectively (P > 0.05). Among Kortilla’s hospital discharge criteria [Citation11], special attention was given to stable vital signs for at least 1 h, no evidence of respiratory depression, patients well-oriented in time and space, the ability of patients to dress and walk, and absence of nausea and vomiting. Post ESWL follow up reported stone clearance occurred in all patients of the 2 groups.
Discussion
Conscious sedation is currently the most commonly used anesthetic technique for elective ESWL, owing to its high patient satisfaction level and its short duration of hospital stay [Citation8]. Alhashemi and Kaki [Citation14] found that dexmedetomidine is an effective and safe drug for conscious sedation during ESWL. Unlike their study evaluating the effects of dexmedetomidine alone, our study was designed to compare the effects of dexmedetomidine and propofol in combination with fentanyl for conscious sedation during ESWL. In this study, patients in the D group reported slower RR, lower OOA/S scores, and lower VAS pain scores. These findings suggest that dexmedetomidine provides advantages over propofol as a sedative analgesic drug during ESWL. The 2 drugs were similar in terms of their effect on MAP, HR, overall pain experiences, patient satisfaction scores, adverse effects, and time to hospital discharge. The results of this study regarding the analgesic effects of dexmedetomidine are in agreement with the findings from both animal and human studies [Citation15–Citation17].
In contrast to the results of our study, Jalowiecki et al. [Citation18] suggested that the use of dexmedetomidine alone at an initial loading dose of 1 μg/kg infused IV over 15 min, followed by an infusion rate of 0.2 μg/kg/h to provide analgesia/sedation for colonoscopy is limited by its many adverse effects, such as hemodynamic instability, a prolonged recovery time, vertigo, nausea, vomiting and a complicated administration regimen. Supplemental fentanyl was required in 47% of patients to achieve a satisfactory level of analgesia. Our study differed in that, for both the D and P groups, we added 1.5 μg/kg of fentanyl intravenously to enhance the analgesic effects of both drugs. In accordance with our results, Belleville et al. [Citation19] reported a significant decrease in RR with the use of dexmedetomidine alone at 1.0, and 2.0 μg/kg IV over 2 min.
Although the RRs decreased in the D group, similar to that seen by Belleville et al. [Citation19] the SpO2 values did not decrease. This may be due to the supplemental O2 and the initial dose of dexmedetomidine given as an infusion and over a longer time (10 min) in our study. Sedative doses of propofol produce minimal depressant effects on tidal volume and minute ventilation, with unchanged end-tidal CO2 tension and arterial blood gases [Citation20]. However, larger doses of propofol can depress the hypoxic ventilatory response [Citation21] and can cause more frequent and longer apnea than barbiturates [Citation22]. In this study, decreases in RR were smaller with propofol than with dexmedetomidine.
We conclude that conscious sedation with dexmedetomidine or propofol in combination with fentanyl for elective ESWL is practical, effective, and safe, provided that monitoring measures are available, and possible respiratory and circulatory complications related to the sedation technique are promptly treated. Sedative, analgesic, and respiratory variables were better with dexmedetomidine than with propofol. Therefore, dexmedetomidine may offer a valuable alternative to propofol.
Conflict of interest
The authors declare no conflict of interest about this study.
Acknowledgment
This study was not supported by any funding source.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- C.BachF.ZamanS.KachrilasP.KumarN.BuchholzJ.MasoodDrugs for pain management in shock wave lithotripsyPain Res Treat2011201110.1155/2011/259426
- Z.GesztesiM.M.RegoP.F.WhiteThe comparative effectiveness of fentanyl and its newer analogs during extracorporeal shock wave lithotripsy under monitored anesthesia careAnesth Analg902000567570
- Z.AybekT.TuranT.YongucC.BozbayO.AtahanO.L.TuncayRequirement of analgesia for extracorporeal shock wave lithotripsy and efficacy of a non-steroidal anti-inflammatory drug: piroxicamEur Urol341998207209
- Y.Y.ChiaProspective and randomized trial of intravenous tenoxicam versus fentanyl and tramadol for analgesia in outpatient extracorporeal lithotripsyActa Anaesthesiol Sin3619981722
- M.A.ClaeysE.GeptsF.CamuHemodynamic changes during anesthesia induced and maintained with propofolBr J Anesth60198839
- K.LeinoL.MildhK.LertolaT.SeppalaO.KirvelaTime course of changes in breathing pattern in morphine- and oxycodone-induced respiratory depressionAnesthesia541999835840
- Z.GesztesiM.M.RegoP.F.WhiteThe comparative effectiveness of fentanyl and its newer analogs during extracorporeal shock wave lithotripsy under monitored anesthesia careAnesth Analg902000567570
- T.G.MonkB.BoureP.F.WhiteS.MeretykR.V.ClaymanComparison of intravenous sedative-analgesic techniques for outpatient immersion lithotripsyAnesth Analg721991616621
- R.M.VennJ.HellR.M.GroundsRespiratory effects of dexmedetomidine in the surgical patient requiring intensive careCrit Care42000302308
- American Society of Anesthesiologists. Continuum of depth of sedation, definition of general anesthesia and levels of sedation/analgesia; October 27, 2004.
- K.KortillaRecovery from outpatient anaesthesia: factors affecting outcomeAnaesthesia50Suppl.19952228
- P.S.GlassM.BloomL.KearseC.RosowP.SebelP.ManbergBispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteersAnesthesiology861997836847
- K.KaygusuzG.GokceS.GursoyS.AyanC.MimarogluY.GultekinA comparison of sedation with dexmedetomidine or propofol during shockwave lithotripsy: a randomized controlled trialAnesth Analg1062008114119
- J.A.AlhashemiA.M.KakiDexmedetomidine in combination with morphine PCA provides superior analgesia for shockwave lithotripsyCan J Anaesth512004342347
- V.KayserJ.DesmeulesG.GuilbaudSystemic clonidine differentially modulates the abnormal reactions to mechanical and thermal stimuli in rats with peripheral mononeuropathyPain601995275285
- M.L.JaakolaM.SalonenR.LehtinenH.ScheininThe analgesic action of dexmedetomidine – a novel alpha 2-adrenoceptor agonist – in healthy volunteersPain461991281285
- T.J.EbertJ.E.HallJ.A.BarneyT.D.UhrichM.D.ColincoThe effects of increasing plasma concentrations of dexmedetomidine in humansAnesthesiology932000382394
- P.JalowieckiR.RudnerM.GonciarzP.KaweckiM.PetelenzP.DziurdzikSole use of dexmedetomidine has limited utility for conscious sedation during outpatient colonoscopyAnesthesiology1032005269273
- J.P.BellevilleD.S.WardB.C.BloorM.MazeEffects of intravenous dexmedetomidine in humans: I. Sedation, ventilation, and metabolic rateAnesthesiology77199211251133
- G.RosaG.ContiP.OrsiEffects of low-dose propofol administration on central respiratory drive, gas exchanges and respiratory patternActa Anesthesiol Scand361992128131
- R.T.BlouinH.A.SeifertH.D.BabencoP.F.ConardJ.B.GrossPropofol depresses the hypoxic ventilatory response during conscious sedation and isohypercapniaAnesthesiology79199311771182
- A.M.Candela TohaJ.Benatar HasserfatyA.Ascorve DominguezInduction and maintenance of anesthesia with propofol and with thiopental-isoflurane: comparative studyRev Esp Anestesiol Reanim381991218221
