Abstract
Background
Children undergoing different surgical procedures and requiring postoperative ventilation need intense analgesia and sedation. This was done using opioids and benzodiazepines with their common side effects as respiratory depression and prolonged sedation.
Aim of the study
To study the efficacy of sedation and time taken form stopping the infusion to extubation using low dose of dexmedetomidine compared with fentanyl sedation in post-operative Pediatric surgical Intensive Care Unit (PICU).
Patients and Methods
A randomized double-blind study involving 50 children undergoing different surgical procedures was performed. The patients were equally divided into two groups, each including 25 patients. One group sedated with fentanyl at 1 μg/kg/h (Fen Group) and the other group sedated with dexmedetomidine at 0.3 μg/kg/h (Dex Group) for 18 h post-operatively with intermittent rescue fentanyl 0.5 μg/kg bolus in the 2 groups as required during endotracheal suctioning. The depth of sedation was assessed using the Ramsay sedation score, the tracheal suctioning score and pediatric intensive care unit sedation score. The time taken from discontinuation of infusion till extubation was recorded.
Results
All sedation scores in the fentanyl and dexmedetomidine groups were comparable. Hemodynamic parameters were comparable between the two groups. Average time (in minutes) required for extubation after stopping the infusions was 136.2 (±54.2 SD) in the Dex group compared with 341.4 (±125.4 SD) in the Fen group. The difference in mean time for extubation was statistically significant. Conclusions: Low dose dexmedetomidine provides adequate sedation for mechanically ventilated children and also early extubation as compared with fentanyl.
Introduction
Pediatric patients in surgical ICU (SICU) require sedation and analgesia, especially if mechanically ventilated, where frequent suctioning is required. Opioids and benzodiazepines are commonly used in Surgical ICU to provide optimum sedation and prevent accidental extubation, in spite of their common side effects e.g. respiratory depression and delayed arousal after the end of the infusion [Citation1].
Dexmedetomidine provides sedation with analgesia through its hypnotic effect and similarity to natural sleep. Central Nervous System (CNS) stimulation of parasympathetic outflow and inhibition of sympathetic outflow from the locus coeruleus in the brain stem plays an important role in sedation and anxiolysis [Citation2]. There are dose dependant bradycardia and hypotension, with doses higher than 0.4 μg/kg [Citation3].
The wide margin of safety of the drug is attributed to its limited respiratory depression effects, with high levels of sedation [Citation4,Citation5].
Dexmedetomidine, alpha2 adrenoceptor agonist, has been used in pediatrics for sedation during variety of clinical situations as non-invasive radiology procedures. Dexmedetomidine has minimal respiratory depressant effects, which makes it the drug of choice as an adjuvant drug for analgesia and sedation in pediatric ICU. It has also a role in preventing postanesthesia emergence delirium and shivering. The loading dose ranges from 0.5 to l μg/kg over 10 min followed by infusion dose of 0.2–5 μg/kg/h [Citation6].
Infusion of Dexmedetomidine makes the patients sedated and easily arousable with hypnosis similar to natural Non-rapid Eye Movement (NREM) sleep [Citation2].
Omitting the loading dose and minimizing the infusion doses to less than 0.4 μg/kg may avoid the occurrence of hypotension and bradycardia [Citation3].
The alpha2 adrenergic agonist, dexmedetomidine, is used in children to produce sedation during radiological procedures, to produce controlled hypotension to decrease intra-operative blood loss and to produce sedation during mechanical ventilation in ICU [Citation7].
Dexmedetomidine infusion at 0.3 μg/kg/h is an effective and well tolerated drug for both mechanically ventilated and spontaneously breathing pediatric patients after cardiac correction surgery. Hypotension and bradycardia were observed with increasing the doses but return back to normal shortly after discontinuation of the infusion [Citation8].
Dexmedetomidine is having good analgesic effects in the postoperative period, reducing morphine requirements by up to 50%, with no adverse effects on respiratory rate, oxygen saturation, arterial partial carbon dioxide tension and arterial pH [Citation9].
The use of different types of opioids via variable roots is associated with dose dependent respiratory depression [Citation10].
Dexmedetomidine, when compared with other sedatives, has both sedative and analgesic effects, reduces agitation and delirium, and has no respiratory depression and minimal cardiovascular effects [Citation11].
It declines noradrenergic output from the locus coeruleus and allows increase in firing of inhibitory neuron (GABA). Centrally acting alpha2 adrenergic agonists also activate central sympatholytic effects, leading to decrease in heart rate and blood pressure. Primary analgesic effects and the increase of opioid-induced analgesia result from the stimulation of alpha2 adrenergic receptor in the dorsal horn of the spinal cord and decrease of substance P release [Citation11,Citation12].
Aim of the study
In this study we compare the efficacy of sedation and time required for extubation for mechanically ventilated children during low dose dexmedetomidine infusion in surgical PICU with that of fentanyl.
Patients and methods
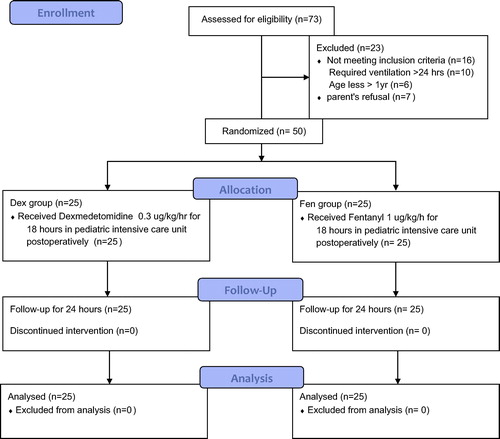
This study was performed in Abo-Elreesh teaching hospital and surgical PICU from September 2013 till December 2014, on 50 children, aged 1–10 years, ASA I&II. After approval from the ethical committee, informed consents were taken from the parents of 50 children to share in this randomized double blinded study. The children were equally divided into 2 groups 25 each, and randomization was done by the closed envelop method. One group sedated with fentanyl at 1 μg/kg/h (Fen Group) and the other group sedated with dexmedetomidine at 0.3 μg/kg/h (Dex Group) for 18 h post-operatively.
Inclusion criteria were pediatric patient’s age group 1–10 years scheduled for abdominal surgery in whom overnight ventilation was anticipated (e.g. huge Hirrsprung’s disease, intestinal resection and diaphragmatic hernia with mechanical compression of the lungs).
Exclusion criteria include patients younger than 1 year, emergency surgeries, patients with severe liver dysfunction, because there is precaution to use Dexmedetomidine in hepatic patients and patients requiring ventilation more than 24 h, to allow us to stop the sedation infusion after 18 h and record the time for extubation.
Preoperative sedation was given to all the patients with intramuscular midazolam 0.1 mg/kg and atropine 0.02 mg/kg.
Induction of anesthesia was done with propofol 3 mg/kg intravenously, after fixation of an intravenous cannula, followed by atracurium 0.5 mg/kg to facilitate endotracheal intubation and fentanyl 2 μg/kg. Anesthesia was maintained with sevoflurane 1 MAC and atracurium at intervals of 20 min.
At arrival to the SICU immediately either dexmedetomidine infusion at a dose of 0.3 μg/kg/h (Dex group) or fentanyl 1 μg/kg/h (Fen group) was started by an anesthesiologist, who is not involved in the study and unaware of the infused drugs [Citation3,Citation13].
The study observer was not aware of the infusion drugs given to the patients of both groups. The infusion was maintained to the next day and then stopped (see ).
The sedation scores such as Ramsay sedation score (RSS), tracheal suctioning score (TSS), Pediatric Intensive Care Unit (PICU) sedation score, and hemodynamic parameters were monitored hourly and recorded at 1, 4, 10, 18 and 24 h respectively [Citation14,Citation15].
.1 Clinical sedation scores used
.1.1 Modified Ramsay sedation score (RSS)
| . | Anxious, agitated, restless. | ||||
| . | Eye opened, cooperative, oriented, tranquil. | ||||
| . | Response (opens eyes) to commands, light touch, normal tone of voice. | ||||
| . | Brisk response to light glabellar tap or loud voice/noise. | ||||
| . | Sluggish response to light glabellar tap or loud voice/noise. | ||||
| . | No response to light glabellar tap or loud voice/noise. | ||||
.1.2 Tracheal suctioning score (TSS)
| . | Patient is restless or distressed when not disturbed. | ||||
| . | Patient is awake and moving, but not distressed if left alone. | ||||
| . | Movement only with nursing care, major limb movement/distressed with tracheal suctioning. | ||||
| . | Cough, grimace or minor limb movement with suctioning. | ||||
| . | No response to tracheal suctioning. | ||||
.1.3 Pediatric Intensive Care Unit (PICU) sedation score
| . | Awake, alert. | ||||
| . | Occasionally drowsy, easy to arouse. | ||||
| . | Frequently drowsy, easy to arouse. | ||||
| . | Somnolent. | ||||
Adequate sedation was met with Ramsay scores of 4 or 5, TSS of 3 or 4 and PICU score of 3. Inadequate sedation was defined as Ramsay scores of 2 or 3, TSS of 1 or 2 and PICU score of 1 or 2, and to those patients 0.5 μg/kg fentanyl was given as rescue sedation in both groups. The sedation infusion was discontinued after 18 h. The time elapsed from stopping the infusion till extubation was recorded.
Extubation criteria include (1) hemodynamic stability, (2) normothermia, (3) awake state, (4) spontaneous breathing at the age appropriate rate, and(5) normocarbia with less than 10 cm H2O pressure support. When those criteria are met patients were ready for weaning from ventilation and extubation was done and nasal cannula with supplemental oxygen was applied.
Hemodynamic compromise severity was classified into 3 degrees as follows:
| 1) | mild: requires no intervention, | ||||
| 2) | moderate: requires reduction of the dose. | ||||
| 3) | sever: requires discontinuation of the infusion. | ||||
.2 Statistical analysis
A power analysis revealed that 25 patients in each group would be demanded to provide 80% power to detect a significant difference between the 2 studied groups. Statistical analysis was performed using SPSS version 10.0 (SPSS ltd, Chicago, IL, U.S.A.). Values are presented as mean (SD) or number (%). Hemodynamic data (as HR and BP) were analyzed using one way analysis of variance (ANOVA) for comparisons between different groups and multiple way ANOVA for comparisons of within group changes. Ordinal categorical data such as sedation scores and need for additional sedation were analyzed with Chi Square test or Fisher’s exact test. Nominal categorical data such as gender, type and duration of surgery were also analyzed with the Chi Square test or Fisher’s exact test. A p value <0.05 was considered to be statistically significant.
Results
The demographic data of the two groups (Fen group and Dex group) were comparable. There was no statistically significant difference in the duration of surgery as seen in .
able 1 Demographic data of the two groups.
The average duration of sedation was around 18 h in both groups. There was no statistically significant difference in the requirement of rescue sedation between the two groups as seen in .
The average time for extubation after cessation of the sedative infusion was 136.2 ± 54.2 min in the Dex group as compared with 341.4 ± 125.4 min in the Fen group, with a P value of <0.001 (statistically significant) as shown in .
able 2 Descriptive analysis of the time for extubation between the two groups.
There was no statistical as well as clinically significant difference in the hemodynamic parameters, i.e. the pulse, systolic blood pressure and diastolic blood pressure, between the two groups.
Even though the heart rate decrease in the Fen group in the first few hours, was <7% to 10% of baseline and did not require any intervention, there was no significant hypotension in either group.
Sedation scores measured at 1, 4, 10, 18 and 24 h, between the two groups were comparable, with no accidental extubation from inadequate sedation. The median with interquartile range of RSS, TSS and PICU sedation score in the Fen and Dex groups was comparable, as shown in .
able 3 Sedation scores in the ICU in postoperative period between the two groups [median (interquartile range)].
Discussion
In pediatric surgical intensive care unit (PSICU) mechanical compression of the lungs from diaphragmatic defects, as in diaphragmatic hernia repair or huge intestinal dilatations, as in Hirrsprung’s disease, when postoperative ventilation is required [Citation7].
Patients require sedation and analgesia to tolerate mechanical ventilation and to prevent inadvertent extubation. Dexmedetomidine by its CNS inhibitory effect on sympathetic outflow from locus coeruleus in the brain stem produces sedation and analgesia [Citation2,Citation5].
The associated decrease in blood pressure and heart rate is due to its sympatholytic effects, and is dose dependent [Citation11].
Dexmedetomidine has minor effect on respiration and, therefore, allows early extubation, [Citation11,Citation13,Citation16] whereas in case of fentanyl, being an opioid, the respiratory depressant effect is the most prominent causing delayed extubation. [Citation17,Citation18].
In this study, we compared the analgesic fentanyl-based sedation (most common agent used in the post-operative intensive care unit because of its hemodynamic stability) with the sedation of the central alpha2 agonist dexmedetomidine; using the RSS, PICU score and TSS as shown in .
In this study, there was a highly significant delay in extubation in the Fen group, the average time being (341.4 ± 125.4 min) as compared with Dex group time (136.2 ± 54.2 min).
This is in line with a retrospective study with post-operative dexmedetomidine infusion in pediatric patients undergoing cardiac surgery. Dexmedetomidine was administered in the post-operative unit at a dose of 0.1–0.5 μg/kg/h for 3–26 h, and the investigators reported successful post-operative sedation in 93% of the patients with absent or minimal pain scores. They also reported that 87% of the patients on dexmedetomidine infusion were easily weaned and extubated [Citation16].
Tobias and Berkenbosch [Citation13] in a prospective randomized study showed that dexmedetomidine at 0.5 μg/kg/h provides more effective sedation and decreased the rescue doses of morphine.
In this study, the sedation in the Dex group was adequate and comparable with the Fen group; the rescue doses of fentanyl required were comparable in both the groups.
In the recent study, the hemodynamic effects were minimal and did not require any intervention. This was probably due to the avoidance of an initial loading dose and also a low infusion dose of 0.3 μg/kg/h in the Dex group [Citation3].
Petroz et al. [Citation12] in a two-center study of pharmacokinetic and pharmacodynamics of dexmedetomidine in children at doses of 2, 4 and 6 μg/kg/h showed that heart rate and systolic blood pressure decreased modestly (<15% and <25%, respectively) during the first hour after dexmedetomidine infusion. The magnitude of decrease in heart rate and blood pressure was proportional to the increase in the dose of dexmedetomidine. At lower doses, the decrease was of modest clinical interest and did not need corrective action.
Bloor et al. [Citation19] and Tobias [Citation11] in their experience with dexmedetomidine found that the potential adverse cardiac and hemodynamic effects of dexmedetomidine, such as bradycardia, sinus arrhythmia and hypotension, occur with the initial loading doses. This is why we preferred to omit the loading dose of dexmedetomidine sedation to prevent any hemodynamic effects in children.
In a study on the effects of increasing doses of dexmedetomidine in children 2, 4, 6 μg/kg/h was infused for 10 min, systolic blood pressure and heart rate decreased respectively, sedation was transiently maintained and respiratory responses were the same [Citation20].
Dexmedetomidine infusion of 1 μg/kg/min for 10 min may produce sedation as long as 4 h after the stoppage of the infusion, but hemodynamic stability was mentioned [Citation21].
In a prospective randomized trial in pediatric intensive care midazolam infusion (0.l mg/kg/h) was compared with dexmedetomidine infusion of 0.25 μg or 0.5 μg/kg/h, respectively. They concluded that dexmedetomidine at a dose of 0.5 μg/kg/h provides better sedation seen with the Ramsay sedation score and the bispectral index and decreases the need for narcotics [Citation22].
Venn et al. [Citation23] in a randomized study showed that dexmedetomidine at an initial loading dose of 1 μg/kg/h over 10 min followed by maintenance dose of 0.7 μg/kg/h provided optimal sedation, but 18 of 66 patients had adverse hemodynamic effects in the form of hypotension or bradycardia, and in 11 of 18 patients the hemodynamic effects were during loading infusion.
Park et al. [Citation24] compared hypnotic-based sedation using propofol and/or midazolam with analgesia-based sedation using remifentanil in a general intensive care unit, and found that analgesia-based sedation provided more satisfactory sedation during mechanical ventilation and also allowed early extubation as compared with hypnotic-based sedation.
Muellejans et al. [Citation25] compared remifentanil versus fentanyl for analgesia-based sedation in the intensive care unit and concluded that analgesia-based sedation with fentanyl or remifentanil was comparable and helped in early extubation of the patients.
Conclusion
Dexmedetomidine in a low dose (0.3 μg/kg/h) provides comparable sedation and stable hemodynamic effects such as that produced by fentanyl. With minimal depression of the respiratory system and sleep-like sedation Dexmedetomidine provides early extubation and can replace fentanyl in pediatric patients postoperatively.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- W.J.GreeleyD.H.BerkowitzA.T.NathanAnesthesia for pediatric cardiac surgeryR.D.MillerMiller’s Anesthesia7th ed.2009Churchill LivingstonePhiladelphia2633
- L.E.NelsonJ.LuT.GuoC.B.SaperN.P.FranksM.MazeThe alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effectsAnesthesiology982003428436
- M.IckeringillY.ShehabiH.AdamsonU.RuettimannDexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: hemodynamic effects and efficacyAnaesth Intensive Care322004741745
- T.TaniguchiY.KidaniH.KanakuraY.TakemotoK.YamamotoEffects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in ratsCrit Care Med32200413221326
- D.A.RosenJ.T.DaumeShort duration large dose dexmedetomidine in a pediatric patient during procedural sedationAnesth Analg10320066869
- H.PhanM.C.NahataCollege of pharmacy, the Ohio State University, Columbus, Ohio, USA. Clinical uses of dexmedetomidine in pediatric patientsPaediatr Drugs10l20084969
- J.D.TobiasJ.W.BerkenboschInitial experience with dexmedetomidine in paediatric-aged patientsPaediatr Anaesth1222002171175
- C.ChrysostomouS.Di FilippoA.M.ManriqueC.G.SchmittR.A.OrrA.CastaE.SuchozaJ.JanoskyP.J.DavisR.MunozUse of dexmedetomidine in children after cardiac and thoracicsurgeryPediatr Crit Care Med722006126131
- R.M.VennJ.HellR.M.GroundsRespiratory effects of dexmedetomidine in the surgical patient requiring intensive careCrit Care452000302308 Epub 2000 Jul 3l
- S.N.KhalilM.E.MatuszczakD.MaposaM.E.BolosH.S.LingadevaruA.Z.ChuangPresurgical fentanyl vs. caudal block and the incidence of adverse respiratory events in children after orchid-pexyPaediatr Anaesth Dec191220091220122510.1111/j. 1460-9592.2009.03164.x.)
- J.D.TobiasDexmedetomidine: applications in pediatric critical care and pediatric anesthesiologyPediatr Crit Care Med82007115131
- G.C.PetrozN.SikichM.JamesH.van DykS.L.ShaferM.SchilyA phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in childrenAnesthesiology105200610981110
- J.D.TobiasJ.W.BerkenboschSedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolamS Med J972004451455
- M.S.ZwassG.A.GregoryPediatric and neonatal intensive careR.D.MillerMiller’s Anesthesia7th ed.2009Churchill LivingstonePhiladelphia2688
- M.A.RamsayT.M.SavegeSimpson BR5 Goodwin R Controlled sedation with alphalaxone–alphadoloneBr Med J21974656659 [PMCID: PMC1613102] [PubMed: 4835444
- C.ChrysostomouS.Di FilippoA.M.ManriqueC.Q.SchmittR.A.OrrA.CastaUse of dexmedetomidine in children after cardiac and thoracic surgeryPediatr Crit Care Med72006126131
- Fukuda K.OpioidsR.D.MillerMiller’s Anesthesia7th ed.2009Churchill LivingstonePhiladelphia782784
- H.GuggenbergerT.H.SchroederR.VontheinH.J.DieterichS.K.ShernanH.K.EltzschigRemifentanil or sufentanil for coronary surgery: comparison of postoperative respiratory impairmentEur J Anaesthesiol232006832840
- B.C.BloorD.S.WardJ.P.BellevilleM.MazeEffects of intravenous dexmedetomidine in humans. II. Hemodynamic changesAnesthesiology77199211341142
- G.C.PetrozN.SikichM.JamesH.van DykS.L.ShaferM.SchilyJ.LermanA phasel, two-centerstudy of the pharmacokinetics and pharmacodynamics of dexmedetomidine in childrenAnesthesiology10562006 Dec10981110
- D.A.RosenJ.T.DaumeShort duration large dose dexmedetomidine in a pediatric patient during procedural sedationAnesth Analg103l20066869
- J.D.TobiasJ.W.BerkenboschSedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolamS Med J9752004 May451455
- R.M.VennC.J.BradshawR.SpencerD.BrealeyE.CaudwellC.NaughtonPreliminary UK experience of dexmedetomidine, a novel agent for postoperative sedatio’n in the intensive care unitAnaesthesia54199911361142
- G.ParkM.LaneS.RogersP.BassettA comparison of hypnotic and analgesic based sedation in a general intensive care unitBr J Anaesth9820077682
- B.MuellejansA.LopezM.H.CrossC.BonomeL.MorrisonA.J.KirkhamRemifentanil versus fentanyl for analgesia based sedation to provide patient comfort in the intensive care unit: a randomized double-blind controlled trial1CritCare82004Rl11