Abstract
Background
Anesthesia for cochlear implantation in pediatrics mandates deliberate hypotension to provide a better surgical field. Dexmedetomidine is α2 adrenoceptor agonist that provides adequate sedation with high cardiovascular stability. We aimed to compare it with fentanyl as an anesthetic adjuvant.
Methods
52 pediatric patients (ASA I or II), undergoing cochlear implantation were randomized into dexmedetomidine (D) group and fentanyl (F) group (n = 26 for each). Anesthesia was induced by I.V. dexmedetomidine in (D) group at a bolus dose of 0.4 μg/kg slowly infused over 10 min, then continuous infusion by a rate of 0.4 μg/kg/h until the end of surgery. In (F) group; anesthesia was induced by I.V. fentanyl at a dose of 1 μg/kg over 10 min, then continuous infusion by a rate of 1 μg/kg/h. This is followed by I.V. propofol and atracurium for both groups. Maintenance was done without additional muscle relaxant to allow monitoring of the facial nerve. Both groups were compared as regards the quality of the surgical field, intraoperative hemodynamics, recovery and discharge time, postoperative pain using objective pain score and the need for rescue analgesics and anti-emetics in postanesthesia care unit (PACU).
Results
Dexmedetomidine group showed a decreased heart rate and mean arterial pressure than fentanyl group. These parameters were significantly decreased compared to the baseline throughout the procedure in D group. The quality of the surgical field was significantly better in D group than in F group. Postoperative pain and complications were not different between the two groups. Recovery and discharge time was significantly shorter for the patients in D group than in F group (p < 0.05).
Conclusion
Dexmedetomidine infusion in cochlear implantation in pediatric patients was better in inducing deliberate hypotension and providing better quality scale of surgical field compared to fentanyl infusion. It allowed rapid recovery from anesthesia and reduced need for pain medication in the PACU.
Introduction
Surgery for cochlear implantation is a great advance in otology for patients with irreversible hearing loss and deaf-mutism but it carries a great challenge to the anesthesiologist [Citation1].
Anesthetic management includes bloodless surgical field to facilitate microsurgery, efficient airway management, careful head positioning to avoid venous obstruction and congestion, limited use of muscle relaxants to facilitate monitoring of the facial nerve by peripheral nerve stimulator, smooth recovery and adequate post-operative care without nausea and vomiting [Citation2].
Controlled hypotension can be achieved by a combination of physical techniques and pharmacologic agents: inhalational anesthetics, opioids, vasodilators, beta blockers, magnesium sulfate and α2 adrenergic agonists [Citation3,Citation4].
Dexmedetomidine is an α2 adrenergic agonist with a sedative and analgesic effect. It does not cause respiratory depression even at supramaximal plasma levels [Citation5]. It suppresses sympathetic activity and decreases airway and circulatory responses during intubation and extubation [Citation6]. Previous reports recommended its use instead of fentanyl to augment anesthesia [Citation7,Citation8].
According to the available data, no study had been published to compare fentanyl and dexmedetomidine in pediatrics undergoing cochlear implantation.
.1 Aim of work
The main objective was to compare fentanyl with dexmedetomidine as regards their efficacy in inducing deliberate hypotension and providing better quality of the surgical field during cochlear implantation. The effect of both drugs on postoperative pain and recovery time was also compared.
Patients and methods
This study was a prospective, randomized, blind study that conducted at Kasr El-Ainy hospital, Cairo University, from April 2012 to March 2014 after approval of ethical committee. Informed consents were obtained from parents or guardians of all children.
Fifty-two pediatric patients of ASA physical status I or II, aged below 8 years and scheduled for elective cochlear implantation, were enrolled in this study. Patients with known allergy to fentanyl or dexmedetomidine were excluded from the study. Also, patients with fever, upper respiratory tract infection, coagulopathy, prolonged QT interval and ventricular arrhythmia were excluded. Also, patients with congenital abnormalities were excluded. Randomization was accomplished by using computerized randomization tables.
All patients were preoperatively assessed by history, physical examination and routine laboratory investigations (CBC, PT, PTT, INR, urea, creatinine, SGPT, SGOT, albumin, bilirubin and serum electrolytes). Cardiological consultation and pre-operative ECG were done. Careful assessment of the airway was done. Careful search for renal, endocrine abnormalities, goiter and hypothyroidism was done.
Solid food was not allowed 6 h before surgery but clear fluids were given for up to 2 h pre-operatively. Children were randomized into dexmedetomidine (D) group and fentanyl (F) group (n = 26 for each). Preparation of dexmedetomidine (Precedex; Abbott Laboratories, North Chicago, Illinois, USA) (vial = 2 ml) 100 μg/ml and fentanyl ampoule 100 μg/2 ml was done. Each drug was diluted with 48 ml of 0.9% NaCl in 50 ml syringe to get a concentration of 4 μg/ml in dexmedetomidine group and 2 μg /ml in fentanyl group.
Demographic data were recorded including age, sex and weight.
On arrival to the operating room; an intravenous catheter was inserted. Monitors were applied: precordial stethoscope, noninvasive automatic blood pressure, pulse oximeter and electrocardiograph. Peripheral nerve stimulator was used to assess recovery from muscle relaxant and to monitor the facial nerve intra-operatively. Premedication with 0.15 mg/kg I.V. dexamethasone was done to prevent postoperative nausea and vomiting. Induction of anesthesia was done by I.V. dexmedetomidine in (D) group at a bolus dose of 0.4 μg /kg slowly infused over 10 min, then continuous infusion by a rate of 0.4 μg/kg/h. until the end of surgery. In (F) group; fentanyl was given at a dose of 1 μg/kg over 10 min, then continuous infusion by a rate of 1 μg/kg/h until the end of surgery. This is followed by propofol 2 mg/kg for both groups. Then, I.V. atracurium at a dose of 0.5 mg per kg was given to facilitate intubation. When T1 is 0%, the patient was intubated by a proper sized cuffed endotracheal tube. Additional doses of atracurium were not administered to allow intraoperative monitoring of the facial nerve.
Anesthesia was maintained using a mixture of O2 and air in a ratio of 1:1 mixture with 2% sevoflurane. Controlled ventilation at a tidal volume of 6 ml/kg was initiated to maintain normocapnia (35–40 mmHg) by adjusting the respiratory rate and guided by the end tidal CO2 monitoring. Body core temperature was measured by oropharyngeal temperature probe and maintained between 36° and 37 °C using heated mattress and warm intravenous fluids at room temperature. Anesthesia was maintained with continuous infusion of the tested drugs. The target blood pressure was a decrease in blood pressure to get the mean blood pressure (MAP = 50–60 mmHg). If the MAP increased above the target, a bolus dose of either dexmedetomidine or fentanyl similar to the induction dose was added. Bradycardia was treated with 0.02 mg/kg I.V. atropine if the HR was 20% lower than the baseline value. Fluids were given at 10 ml/kg/h in the form of dextrose 5% and normal saline at a ratio of 1:1. At the end of the procedure, the patient was extubated under deep anesthesia to avoid coughing (which may cause dislodgement of the electrode array of the implant) and transferred to the recovery room.
.1 Intra-operative data recorded
| . | Heart rate (HR) and mean arterial blood pressure (MAP). These data were recorded before induction (baseline), 1 min after induction, 1 min after intubation then every 15 min till the end of the operation. | ||||
| . | Total dose of dexmedetomidine and fentanyl. | ||||
| . | Total time of the operation. | ||||
| . | Quality scale: | ||||
The surgeon who was blinded of the selected hypotensive agent was asked to assess the quality of the surgical field according to the quality scale proposed by Fromme and colleagues [Citation9]:
0 = no bleeding.
1 = slight bleeding – blood evacuation not necessary.
2 = slight bleeding – sometimes blood has to be evacuated.
3 = low bleeding – blood has to be often evacuated. Operative field is visible for some seconds after evacuation.
4 = average bleeding – blood has to be often evacuated. Operative field is visible only right after evacuation.
5 = high bleeding – constant blood evacuation is needed. Sometimes, bleeding exceeds evacuation. Surgery is hardly possible.
Postoperatively, both recovery time and discharge time were recorded for all patients. Recovery time was defined as the period of time from discontinuation of sevoflurane till achieving a modified Aldrete recovery score of at least 9. Discharge time was defined as the time from the end of the procedure until the child fulfilled the discharge criteria from PACU.
The criteria of discharge were returning of vital signs and level of consciousness to baseline, and ability to ambulate without help and to tolerate clear fluids without nausea and vomiting.
Postprocedural recovery was evaluated using a modified Aldrete score [Citation10] at 10 min at the recovery room. Postprocedural pain was assessed every 10 min in the recovery room using Objective Pain Scale (OPS) [Citation11]. Diclofenac suppository (12.5 or 25 mg) was given if OPS was ⩾4. It was given according to the nearest dose guided by body weight (2 mg/kg).
Postoperative nausea and vomiting were monitored for 24 h. Intravenous ondansetron (0.1 mg per kg) was given if nausea and vomiting had occurred. Number of patients who suffered from apnea was recorded. The anesthetist who was recording the intra-operative and postoperative data did not share in preparing or giving the selected agent.
.2 Sample size calculation
Based on two-tailed α error probability of 0.05 and β error probability of 0.2 (power of 80%), a total sample size of 52 patients equally allocated into two groups was required to detect a presumed minimum clinically significant decrease of 20% in intra-operative mean arterial blood pressure (effect size d of 0.8). Statistical power calculations were performed using computer program G∗Power 3 for Windows (Franz Faul, Universität Kiel, Germany).
.3 Statistical analysis
Obtained data were presented as mean ± SD, ranges, numbers and percentages as appropriate. Nominal variables were analyzed using Chi-squared (χ2) test or Fischer exact test as appropriate. Continuous variables were analyzed using unpaired Student’s t-test or univariate two-group repeated measures analysis of variance (ANOVA) with post hoc Dunnett’s test as appropriate. Nominal and non-normally distributed variables were analyzed using Mann–Whitney U test. Statistical analysis was performed using SPSS (Version 20, 2011). P value < 0.05 was considered statistically significant.
Results
Fifty-two patients were enrolled (26 patients in each group) and completed the study.
| #x2013; | There was no statistically significant difference between both groups regarding the patients’ demographic data and operative time (). | ||||
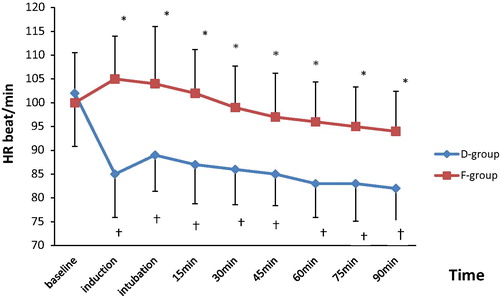
| #x2013; | Intra-operative heart rate was statistically significantly lower in D group than in F group at all time periods (P < 0.05). In (D) group, there was a significant reduction in heart rate values in comparison with the baseline value at all time periods while in (F) group, the difference was not significant (). | ||||
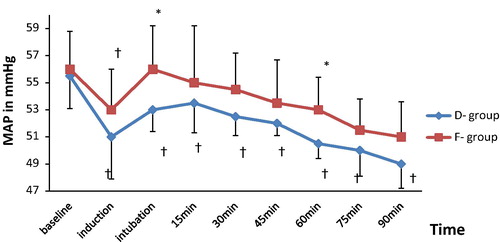
| #x2013; | Intra-operative mean blood pressure (MAP) was lower in D group than in F group at all time periods. This decrease was statistically significant at 1 min after intubation and at 60 min but no significant difference was observed between both groups during the remaining time periods. The MAP decreased significantly from the baseline value at all time periods in (D) group while in (F) group the decrease in MAP from the baseline was not significant during all time periods except at 1 min after induction (). | ||||
| #x2013; | The quality of the surgical field intra-operatively was significantly better in dexmedetomidine group (). | ||||
| #x2013; | Total dose of the drug used intra-operatively was 78.28 ± 22.05 μg for fentanyl and 26.22 ± 6.30 μg for dexmedetomidine. 2 patients in dexmedetomidine group required a bolus dose (given once), while in fentanyl group 6 patients required a bolus dose (given once). | ||||
| #x2013; | Regarding recovery and discharge time, dexmedetomidine group showed significantly shorter recovery time [9.5 ± 2.46] min versus [12.28 ± 3.47] min in F-group, and significantly shorter discharge time [23.46 ± 4.29] min versus [28.34 ± 5.78] min in F-group. | ||||
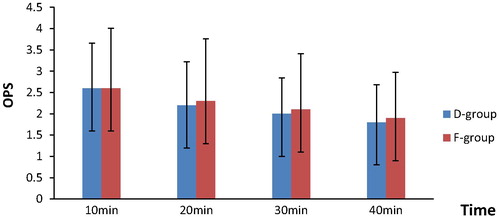
| #x2013; | There was no statistically significant difference between both groups regarding the objective pain score (every 10 min from 10 to 40 min) (). Two patients of D group and seven patients of F group had required bolus doses of rectal diclofenac at 10 min at PACU. It was given once. | ||||
| #x2013; | There was no statistically significant difference between both groups regarding modified Aldrete score (), and the need for anti-emetics in PACU (Five patients in fentanyl group required anti-emetics versus two patients in dexmedetomidine group). No cases of apnea were reported in the study groups. | ||||
able 1 Demographic data and operative time.
able 2 Comparison between dexmedetomidine group and fentanyl group as regards quality scale for surgical field intra-operatively.
able 3 Comparison between fentanyl group and dexmedetomidine group as regards modified Aldrete score.
Discussion
Dexmedetomidine is a potent α2 adrenergic agonist with a distribution half-life of 8 min and a terminal half-life of 3.5 h. Its short half-life provides easy titration, quick recovery and less adverse events related to prolonged sedation. It provides adequate sedation with high cardiovascular and respiratory stability [Citation12,Citation13]. α2-adrenoceptors exist on the dorsal horn neurons of the spinal cord and can release endogenous opiate compounds. Thus, α2-adrenoceptor agonists may be used in pain management and may decrease intra-operative opioid requirements, similar to clonidine [Citation14].
The main finding in this study was that dexmedetomidine showed a significant reduction in intra-operative HR and MAP more than fentanyl. However, the intra-operative reduction in hemodynamic parameters (MAP and HR) in both groups was within 20% from baseline values. It also showed that dexmedetomidine has significantly better surgical quality scale, shorter recovery and discharge time than fentanyl.
The use of dexmedetomidine in pediatric population is limited. Mason et al. [Citation15] were the first who studied the sedative effect of dexmedetomidine on pediatric patients for radiological imaging studies. They reported that dexmedetomidine produced a reduction of HR and MAP which was clinically acceptable for the pediatric age group. These findings coincide with the results of the present study. On the other hand, Koroglu et al. [Citation13] noticed that dexmedetomidine produced a reduction of HR only in comparison with propofol in children undergoing MRI study.
In line with the current study, Tanskanen et al. [Citation16] reported that dexmedetomidine was an excellent anesthetic adjuvant because of the perioperative hemodynamic stability and the faster tracheal intubation that obtained in comparison with fentanyl in patients undergoing brain surgery.
Also, Feld et al. [Citation7] compared dexmedetomidine with fentanyl in bariatric surgery. They reported that dexmedetomidine decreased sympathovagal balance and heart rate intra-operatively more than fentanyl.
Bulow et al. [Citation17] concluded that dexmedetomidine provided better hemodynamic stability than remifentanil in patients undergoing invasive video gynecologic surgical procedures. They observed a prolonged recovery time in patients received dexmedetomidine when compared with those received remifentanil. Remifentanil has an elimination half-life of 7 min and degradation is done by blood and tissue esterase. So, remifentanil infusion does not cause overhanging effects and is much shorter than fentanyl. This could explain their results.
Ali et al. [Citation18] had compared dexmedetomidine with fentanyl in pediatric patients undergoing extracorporeal shock wave lithotripsy and reported that the MAP and HR were significantly decreased compared to the baseline throughout the procedure in both groups. These results are consistent with the present study.
Turgut et al. [Citation19] reported that MAP values were significantly higher in dexmedetomidine group than in fentanyl group only after intubation, while they were significantly lower in dexmedetomidine group than in fentanyl group before and after extubation during lumbar laminectomy surgery. There was no statistically significant difference in HR between groups. In contrast to the present study, they noticed a comparable recovery and discharge times when comparing fentanyl and dexmedetomidine. This could be due to the smaller loading and maintenance doses (fentanyl: 0.64 ± 0.06 μg/kg−1 h, dexmedetomidine 0.31 ± 0.08 μg/kg−1 h) they have used.
This study found no significant difference between both groups as regards postoperative nausea and vomiting, and objective pain score. Also, dexmedetomidine decreased the need for pain medication in the PACU.
In accordance with these findings, Feld et al. [Citation7] reported that dexmedetomidine provided stable postoperative analgesia, thus reducing the use of morphine in the postoperative period when comparing fentanyl and dexmedetomidine combined with desflurane for bariatric surgery.
However, Ali et al. [Citation18] reported significantly higher incidence of nausea and vomiting in pediatric patients receiving fentanyl in comparison with those receiving dexmedetomidine during extracorporeal shock wave lithotripsy. Also, the same results were reported by Turgut et al. [Citation19] in adult patients undergoing lumbar laminectomy.
As a limitation of this study, there was no control group in which propofol was used alone, as it would be unethical; also a subjective scoring system was used to evaluate the quality of the surgical field and the lack of BIS monitor to assess depth of anesthesia. Future studies are needed to compare the efficacy of dexmedetomidine with other commonly used agents during cochlear implantation in pediatrics.
Conclusion
Dexmedetomidine infusion in cochlear implantation in pediatric patients was better in inducing deliberate hypotension and providing better quality scale of surgical field compared to fentanyl infusion. It allowed rapid recovery from anesthesia and reduced need for pain medication in the PACU.
Conflict of interest
No conflict of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- C.B.PedersenU.JochumsenS.MadsenResults and experiences with 55 cochlear implantationsUgeskr Laeger16240200053465350
- E.G.MorganM.S.MikhailM.J.Murray5th ed.Clinical anesthesiologyvol. 372006Lange Medical Books/McGraw-HillNew York
- C.S.DegouteControlled hypotension: a guide to drug choiceDrugs677200710531076
- J.H.RyuI.S.SohnS.H.DoControlled hypotension for middle ear surgery: a comparison between remifentanil and magnesium sulphateBr J Anaesth10342009490495
- Y.W.HsuL.I.CortinezK.M.RobertsonDexmedetomidine pharmacodynamics: Part I Crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteersAnesthesiology101200410661076
- G.GulerA.AkınZ.TosunA Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubationActa Anesthesiol Scand49200510881091
- J.M.FeldW.E.HoffmanM.M.StechertFentanyl or dexmedetomidine combined with desflurane for bariatric surgeryJ Clin Anesth1820062428
- R.E.HoferJ.SprungM.G.SarrAnesthesia for a patient with morbid obesity using dexmedetomidine without narcoticsCan J Anaesth5222005176180
- G.A.FrommeR.A.MackenzieA.B.GouldJr.Controlled hypotension for orthognathic surgeryAnesth Analg6561986683686
- J.A.AldreteThe post anesthesia recovery score revisitedJ Clin Anesth719958991
- P.KundraK.DeepalakshmiM.RavishankarPreemptive caudal bupivacaine and morphine for postoperative analgesia in childrenAnesthesia Analg87119985256
- L.E.NelsonJ.LuT.GuoThe alpha 2 adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effectsAnesthesiology982003428436
- A.KorogluS.DemirbilekH.TeksanSedative, hemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary resultsBr J Anaesth9462005821824
- M.XuV.K.KontinenE.KalsoEffects of radolmidine, a novel alpha 2-adrenergic agonist compared with dexmedetomidine in different pain models in the ratAnesthesiology932000473481
- K.P.MasonS.E.ZgleszewskiJ.L.DeardenDexmedetomidine for pediatric sedation for computed tomography imaging studiesAnesth Analg10320065762
- P.E.TanskanenJ.V.KyttaT.T.RandellDexmedetomidine as an anesthetic adjuvant in patients undergoing intracranial tumour surgery: a double-blind, randomized and placebo-controlled studyBr J Anaesth9762006658665
- N.M.BulowN.V.BarbosaJ.B.RochaOpiod consumption in total intravenous anesthesia is reduced with dexmedetomidine: a comparative study with remifentanil in gynecologic video laparoscopic surgeryJ Clin Anesth192007280285
- A.AliM.El GhoneimyDexmedetomidine versus fentanyl as adjuvant to propofol: comparative study in children undergoing extracorporeal shock wave lithotripsyEuro J Anesthesiol27201010581064
- N.TurgutA.TurkmenS.GokkayaDexmedetomidine-based versus fentanyl-based total intravenous anesthesia for lumber laminectomyMinerva Anesthesiol742008469474
