Abstract
Background
To reduce intraoperative use of volatile anesthetics, a successful caudal blockade affords the anesthesiologist this opportunity. The use of a narcotic-sparing approach may benefit the patient, with providing a better postoperative course with less nausea.
Aim of the work
To compare the effects of plain levobupivacaine 0.25% 1 ml/kg and levobupivacaine 0.25% 1 ml/kg plus nalbuphine 0.1 mg/kg single-shot caudal epidural for perioperative pain relief in children undergoing surgeries of lower half of the body.
Patients and methods
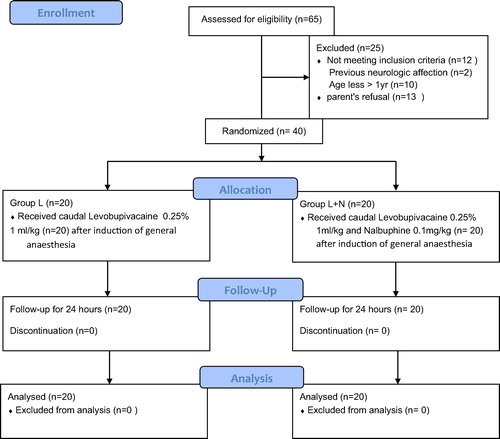
The study was conducted in Abou El-Reesh pediatric hospital, Cairo University, after approval of ethical committee and obtaining consent from parents on 40 patient aged 1–9 years scheduled for surgeries of lower half of the body. (Group L, n = 20): Caudal block was done in this group using levobupivacaine 0.25% with the dose of 1 ml/kg after induction of general anesthesia. (Group L + N, n = 20): Caudal block was done in this group using levobupivacaine 0.25% with the dose of 1 ml/kg and nalbuphine 0.1 mg/kg after induction of general anesthesia.
Results
The time to first analgesia was significantly longer in Group L + N (P < 0.01) than that in the other group. The mean time for first rescue analgesia was 5.9 ± 1.0 h in Group L compared to that in Group L + N, which was 11.2 ± 1.6 h. Comparing the pain scores (AIIMS pain score) of the two groups at 2, 4, 6, 12 and 24 h postoperatively revealed that there was significant difference between Group L + N and Group L at 4, 6 and 12 h with higher pain scores in the (Group L) than in the other Group (L + N). This shows that the duration of analgesia in the (L + N) group was longer than the other group. The results show there was difference in the sedation score between the two groups in the 1st hour postoperative. The L + N group had higher sedation scores at 30 min and at 1 h postoperative.
Conclusion
Caudal epidural nalbuphine is safe in pediatric surgeries including the lower half of the body and effectively reduces postoperative pain. However it may cause early postoperative sedation, yet without respiratory depression.
Introduction
To reduce intraoperative use of volatile anesthetics, a successful caudal blockade affords the anesthesiologist this opportunity. The use of a narcotic-sparing approach may benefit the patient, with providing a better postoperative course with less nausea and vomiting [Citation1]. The quality and level of the caudal blockade are dependent on the agent’s dose, volume, and type of the injected drugs. The relatively short duration of postoperative analgesia is one of the major limitations of the single-injection technique. The most frequently used method to further prolong postoperative analgesia following single-injection caudal block, is to add different adjuvants to the local anesthetics [Citation2]. Prolongation of anesthesia can be obtained by adding various types of adjuvants, such as opioids or non-opioids such as clonidine, neostigmine, ketamine, midazolam and with varying concentrations to achieve different degrees of success [Citation3,Citation4].
Nalbuphine hydrochloride is a synthetic opioid agonist – antagonist analgesic of the phenanthrene series. Adding Nalbuphine to epidural analgesic agents, provides an increase in the efficacy and the duration of postoperative analgesia [Citation5]. The use of nalbuphine as a sole analgesic agent provides satisfactory cover of mild to moderate types of pain with a low incidence of side effects. The ceiling effect of nalbuphine with increasing the dose, which prevents it from covering the most severe pain, also prevents unwanted sedation and respiratory depression. Nalbuphine provides an increased safety margin, when compared to μ-agonists. When nalbuphine is used concurrently with μ-agonists (e.g. morphine and fentanyl), the benefits of both μ- and κ-analgesia can be obtained, with decreasing the severity of the common μ-agonist side effects (itching, nausea/vomiting, urinary retention, constipation, respiratory depression and prolonged sedation) [Citation6].
Regional anesthesia in children is of utmost importance to potentiate the effect of the general anesthesia and to prevent pain before it is initiated. The use of analgesic combination is best limited to the one, which does not produce respiratory depression or vomiting [Citation7].
The levorotatory isomers of bupivacaine were shown to have a safer pharmacological profile with less cardiac and neurotoxic adverse effects. The decreased toxicity of levobupivacaine is attributed to its faster protein binding rate. Levobupivacaine produces subarachnoid block similar to the sensory and motor effects and recovery of bupivacaine with earlier regression of its motor block. Intrathecal administration of 15 mg of levobupivacaine provides an adequate sensory and motor block lasting for approximately 6.5 h, while smaller doses (i.e., 5–10 mg) are used in day-case surgeries. Low concentrations of levobupivacaine may be favorable for ambulatory surgery. The addition of opioids provides a dose sparing effect of levobupivacaine, which improves the quality of the block with less hemodynamic changes [Citation8].
The dose of levobupivacaine for infant spinal anesthesia is 1 mg/kg of isobaric 0.5% bupivacaine and ropivacaine and 1.2 mg/kg of isobaric 0.5% levobupivacaine. The recommended dose of levobupivacaine for effective caudal anesthesia has been studied to be 2.5 mg/kg. Post-operative epidural infusions of 0.125% levobupivacaine or ropivacaine in pediatric patients produce significantly less motor blockade with equal analgesia as compared to a similar infusion of bupivacaine. It is important to note that toxicity of local anesthetics may be potentiated in patients with hepatic or renal affection, respiratory diseases and pre-existing heart conditions. The drug toxicity may be potentiated with hypoxia. However, the most common cause of the toxicity is inadvertent intravascular injection [Citation9].
.1 The rational of this work
To find out that the addition of nalbuphine to the local anesthetic will prolong the duration of postoperative analgesia and reduce the need for rescue analgesia.
.2 Aim of the work
To compare the effects of plain levobupivacaine 0.25% 1 ml/kg and levobupivacaine 0.25% 1 ml/kg plus nalbuphine 0.1 mg/kg single-shot caudal epidural for perioperative and postoperative pain relief in children undergoing surgeries on lower half of the body. To measure the effect of nalbuphine in prolonging the time for first rescue analgesia, so reducing the use of narcotics.
Patients and methods
This double-blinded, randomized, controlled study is designed to explore and compare the effect of adding nalbuphine to levobupivacaine for treating post-operative pain in children undergoing pelvi-abdominal surgeries. Our ultimate goal is to identify the time during which the child remains pain free post-operative before the first rescue analgesia is given.
The study was conducted in Abou El-Reesh pediatric hospital, Cairo University, after approval of ethical committee and obtaining consent from parents on 40 patient aged 1–9 years scheduled for pelvi-abdominal surgeries.
.1 Exclusion criteria
The exclusion criteria were parent refusal, local infection at site of caudal block, allergy to local anesthetics, bleeding tendency and pre-existing neurological or spinal diseases.
The patients were randomly divided using computer generated number and concealed using sequentially numbered, sealed opaque envelope technique into two equal groups (each 20 patients): Group L and Group L + N.
(Group L): Caudal block was done in this group using levobupivacaine 0.25% with the dose of 1 ml/kg after induction of general anesthesia.
(Group L+N): Caudal block was done in this group using levobupivacaine 0.25% with the dose of 1 ml/kg and nalbuphine 0.1 mg/kg after induction of general anesthesia.
None of the patients received premedication.
.2 Induction of anesthesia
On arrival to the operating room, all patients were connected to the monitor including ECG, non-invasive blood pressure and pulse oximetry, induction of anesthesia was initiated by inhalational route using Sevoflurane 4%, and then an intravenous cannula was inserted and injection of atropine 0.01 mg/kg was administrated. Anesthesia was maintained with isoflurane 2–3% and 100% oxygen with spontaneous breathing. No sedatives or opioids will be administered during the procedure. Then the caudal block was done according to their group.
During surgery, adequate intraoperative analgesia will be defined by hemodynamic stability, as evidenced by the absence of an increase in the heart rate or a mean arterial blood pressure greater than 15% compared to the baseline values obtained before skin incision. An increase in the HR or MAP within 15 min of skin incision indicates failure of caudal anesthesia. If the readings were increased by >15%, the child will receive a rescue opioid in the form of fentanyl 0.5 μg/kg, because analgesia will be considered inadequate.
Then the patient was transported to the recovery room with routine monitoring to the heart rate, non-invasive arterial blood pressure, oxygen saturation and pain scoring. All the measurements were recorded by the anesthetist and the recovery room doctors, who were blinded to the drug combination given to the patients.
.3 The study outcome will be measured as follows
.3.1 Primary outcome measure
The primary outcome measure was time to first request for rescue postoperative analgesia.
.3.2 Secondary outcome measures
| . | Hemodynamics: Heart rate, systolic and diastolic blood pressure and oxygen saturation recorded before the block, every 10 min after caudal block and 15 min after recovery. | ||||
| . | Pain score: Pain score will be recorded after extubation at 2, 4, 6, 12, and 24 h in the recovery room by All India Institute of Medical Sciences (AIIMS) [Citation10] pain discomfort scale which measures five variables: respiratory rate, heart rate, discomfort, cry and pain at the site of operation. A lower score is associated with less pain. The duration of absolute analgesia will be defined as the time from caudal injection until a pain score was ⩾2 . | ||||
Rescue analgesia will be given for a pain discomfort scale score ⩾4 in the form of I.V Perfalgan (15 mg/kg) and if not enough Diclofenac suppositories (1–2 mg/kg) will be added.
| . | Sedation score: Assessment of sedation will be done at 30 min, 1 and 2 h by using an objective score based on eye-opening (eyes open spontaneously = 0, eyes open in response to verbal stimulation = 1, eyes open in response to physical stimulation = 2) [Citation11]. | ||||
able 1 AIIMS pain discomfort scale [Citation10].
Any complications observed in the postoperative period such as nausea, vomiting, urinary retention, respiratory depression or hemodynamic instability will be recorded.
Statistical analysis
The sample size of the study was calculated using the G∗ Power application according to the results of a pilot study, in which we found an increase in the time to first request for rescue analgesia by a mean difference of 1.6 h and a SD difference of 0.8 h; it was found that 14 patients are required to achieve 0.9 power, so we decided to include 20 patients in each group to allow for the dropouts. The data of the results were analyzed and reported as mean (±SD), number (%) or median (range) hemodynamic data were analyzed using one-way analysis of variants (ANOVA) for comparisons between different groups and multiple way ANOVA for comparisons of within group changes. Ordinal categorical data such as sedation scores and need for additional sedation were analyzed with Chi Square test or Fisher’s exact test. Nominal categorical data as gender and age were also analyzed with the Chi Square test or Fisher’s exact test. For all statistical tests done, a p value < 0.05 indicates significant difference ().
Results
The demographic data of the patients didn’t show statistical significance between the two groups as shown in .
able 2 Patient’s demographic data numbers and mean (SD).
There was no statistically significant difference among the two groups as regards heart rate and mean arterial blood pressure at different times. There was no need for extra analgesia intra-operative (in the form of fentanyl 0.5 μg/kg I.V.) among all patients in the two groups. The results show no statistically significant difference among the two groups as regards oxygen saturation at different times.
The time to first administration of rescue analgesia was 5.9 ± 1.0 h in Group L, as three patients requested the first rescue analgesia at 7 h post-op, while six patients did at 6 h, seven patients requested at 5 h and four patients at 4 h.
In Group L + N the mean time for first rescue analgesia was 11.2 ± 1.6 h as six patients required rescue analgesia at 9 h post-op., three patients at 10 h, four patients at 11 h, four patients at 12 h and three patients at 13 h post-operative.
The time to first analgesia was significantly longer in Group L + N (P < 0.01) than that in the other group as shown in .
able 3 Time for first analgesia in postoperative hours among the 2 groups.
Comparing the pain scores (AIIMS Pain Score) of the two groups at 2, 4, 6, 12 and 24 h postoperatively revealed that there was significant difference between Group L + N and Group L at 4, 6 and 12 h with higher pain scores in the (Group L) than in the other group (L + N). This shows that the duration of analgesia in the (L + N) group was longer than the other group as demonstrated in .
able 4 AIIMS pain discomfort scale among the 2 groups.
The results show there was difference in the sedation score between the two groups in the 1st hour postoperative. The L + N group had higher sedation scores at 30 min, at 1, and 2 h postoperatively as shown in .
able 5 Sedation scores among the 2 groups.
There was no significant difference between the two groups in the time to first void. In Group L the time was 96.3 ± 17.7 min and in Group L + N it was 104.6 ± 14.4 min post-operatively. None of the patients in the two groups had motor block on emergence from anesthesia. No child required bladder catheterization.
Discussion
Caudal anesthesia is a useful adjuvant to general anesthesia in children. It provides analgesia during and following operations involving the lower half of the body. The use of an adjuvant to the local anesthetic prolongs and intensifies its effect.
This study was held at Cairo University Pediatric Hospital (Abu El Reesh). Forty patients were enrolled in the form of two groups with patient’s age ranging from 1 to 9 years. Group L (levobupivacaine only) was 20 patients and Group L + N (levobupivacaine plus nalbuphine) was 20 patients.
The study demonstrated that adding nalbuphine to local anesthetic (levobupivacaine) was effective as evidenced by decreased rescue postoperative analgesia and lower pain scores.
Breschan and colleagues [Citation12] compared the analgesic efficacy of levobupivacaine, ropivacaine and bupivacaine caudal block in pediatric patients undergoing caudal blockade. Children aged 1–7 years, undergo either inguinal hernia repair or orchidopexy. Their results were consistent with the results of the current study in the effectiveness of levobupivacaine in post-operative analgesia, but differs in using concentration of 0.2% levobupivacaine instead of 0.25% and in using Children’s and Infant’s Postoperative Pain Scale observational scale.
Locatelli and colleagues [Citation13] compared the effect of levobupivacaine 0.25%, ropivacaine 0.25% and bupivacaine 0.25% by the caudal route in children less than 10 yr old scheduled for elective sub-umbilical surgery. The results showed that the effective analgesia in children during the operation was similar among groups. Bupivacaine produced a significant incidence of residual motor block compared with levobupivacaine or ropivacaine at wake-up (P < 0.01).
The results of the study were consistent with the current study in the analgesic effect of levobupivacaine post-operative and in the absence of motor block after caudal levobupivacaine but it differs in and in using the Children’s and Infants’ Postoperative Pain Scale (CHEOPS) for assessment of post-operative pain.
Yildiz and colleagues [Citation14] studied and compared effects of levobupivacaine-tramadol combination for caudal block in children. The results showed that the addition of tramadol to levobupivacaine prolonged the duration of analgesia significantly (P < 0.01). No signs of motor block were recorded after the first postoperative hour in any patient.
The results of the study were consistent with the current study in the analgesic effect of levobupivacaine post-operative and in the absence of motor block after caudal levobupivacaine but differs in using lower concentration of levobupivacaine (0.125%), and in using the Children’s and Infants’ Postoperative Pain Scale (CHEOPS) for assessment of post-operative pain
Kaya and colleagues [Citation15] compared the effects of caudally administered levobupivacaine 0.25% and bupivacaine 0.25% on pain and motor block in children undergoing circumcision surgery. Patients’ ages ranging from 1 to 10 years underwent elective circumcision surgery. Results showed that, the mean children’s and infant’s postoperative pain scale of Group B was significantly lower than that of Group L (p < 0.001).
The results were different from that in the current study in using volume of epidural levobupivacaine 0.5 ml/kg compared to 1 ml/kg in this study, using postoperative pain assessment by children’s and infant’s postoperative pain scale. But was comparable with our study in confirming that the analgesic potency of levobupivacaine and in the absence of motor block after caudal levobupivacaine. Limitation of this study is the small sample size, so further studies will be needed with larger number of patients.
Conclusion
From the results of the present study it is concluded that caudal epidural nalbuphine is safe in pediatric surgeries in the lower half of the body and effectively reduces postoperative pain. However it may cause early postoperative sedation, yet without respiratory depression.
Conflict of interest
There is no conflict of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- D.A.H.de BeerM.L.ThomasCaudal additives in children: solution or problems?Br J Anaesth902003487498
- J.J.LeeA.P.RubinComparison of a bupivacaine-clonidine mixture with plain bupivacaine for caudal analgesia in childrenBr J Anaesth721994258262
- M.NaguibA.M.SharifM.SerajM.el GammalA.A.DawlatlyKetamine for caudal analgesia in children: comparison with caudal bupivacaineBr J Anaesth671991559564
- M.AbdulatifM.El-SanabaryCaudal neostigmine, bupivacaine, and their combination for postoperative pain management after hypospadius surgery in childrenAnesth Analg95200214
- M.NaguibM.El GammerY.S.ElhattabM.SerajMidazolam for caudal analgesia in children: comparison with caudal bupivacaineCan J Anaesth421995758764
- J.C.EisenachR.CarpenterR.CurryAnalgesia from a peripherally active-opioid receptors agonist in patients with chronic pancreatitisPain10120038995
- E.N.ArmitageRegional anesthesia in pediarticsActa Anesthesiol Belg3931988191195
- S.J.Singh BajwaJasleenKaurClinical profile of levobupivacaine in regional anesthesia: a systematic reviewJ Anaesthesiol, Clin Pharmacol2013
- M.F.MulroySystemic toxicity and cardiotoxicity from local anesthetics: incidence and preventive measuresReg Anaesth Pain Med2762002556561
- G.P.DurejaHand book of pain management1st ed.2004ElsevierIndia 36
- A.H.ChoudhuriP.DharmaniN.KumarlA.PrakashComparison of caudal epidural bupivacaine with bupivacaine plus tramadol and bupivacaine plus ketamine for postoperative analgesia in childrenAnaesthesia Intensive Care362008174179
- C.BreschanR.JostR.KrumpholzF.SchaumbergerH.StettnerP.MarhoferR.LikarA prospective study comparing the analgesic efficacy of levobupivacaine, ropivacaine and bupivacaine in pediatric patients undergoing caudal blockadePaediatr Anaesth1542005301306
- B.LocatelliP.IngelmoV.SonzognA.ZanellaV.GattiA.SpottiRandomized, double-blind, phase III, controlled trial comparing levobupivacaine 0.25%, ropivacaine 0.25% and bupivacaine 0.25% by the caudal route in childrenBr J Anaesth9432005366371
- T.S.YildizD.OzdamarF.BagusM.SolakK.TokerLevobupivacaine-tramadol combination for caudal block in children: a randomized, double-blinded, prospective studyPaediatr Anaesth2062010524529
- Z.KayaM.SurenS.AriciS.KaramanH.TaparF.ErdmirProspective, randomized, double-blinded comparison of the effects of caudally administered levobupivacaine 0.25% and bupivacaine 0.25% on pain and motor block in children undergoing circumcision surgeryEur Rev Med Pharmacol Sci16201220142020