Abstract
Objective
The rapid emergence and recovery from sevoflurane anesthesia is associated with a high incidence of emergence agitation in children, ranging up to 80% (Yan-lin and Hong-bo, 2010). Both ketofol and dexmedetomidine have been shown to successfully reduce the incidence and severity of EA, if administered at the end of sevoflurane anesthesia. However, it was not determined which agent has better efficacy. The purpose of this study was to compare the effectiveness of ketofol and dexmedetomidine, given 10 min before the end of surgery, in preventing EA.
Patients and methods
Ninety pediatric patients, aged 3–6 years old, American Society of Anesthesiologists I or II, and undergoing orthopedic surgeries under sevoflurane-based anesthesia were recruited into the study. They were randomly assigned to one of three equal groups: Group K, received ketofol (ketamine 0.25 mg/kg and propofol 1 mg/kg); Group D, received dexmedetomidine 0.3 μg/kg and Group C, received 0.9% normal saline. The study drugs were given 10 min before the end of surgery. In postanesthesia care unit, incidence of EA was evaluated with Aono’s four point scale and the severity of EA was assessed using Paediatric Anesthesia Emergence Delirium scale upon awakening (T0), after 10 min (T10), 20 min (T20), and 30 min (T30). Extubation time, emergence time and time of first analgesic requirement were also recorded.
Results
There were no significant differences in demographic data, duration of surgery or sevoflurane exposure among the three groups. The incidence of EA in group K and group D was similar and significantly lower than that in group C at T0, T10, and T20. The incidence of EA decreased significantly over time in all groups. The severity of EA was significantly lower in groups K and D than in group C at T0, T10 and T20. Time to extubation and to get modified Aldrete score ⩾9 was significantly longer in group D than that in group K. In comparison to control group, group K had longer extubation time and time to get modified Aldrete score ⩾9. The time of first analgesic requirement was significantly longer in group K than that of group D. Both groups K and D provided better analgesic effect in early postoperative period when compared to control group.
Conclusion
We found that ketofol in a dose (propofol 1 mg/kg in a combination with ketamine 0.25 mg/kg) was as effective as dexmedetomidine in a dose of 0.3 μg/kg for prevention of EA, but with better analgesic effect and without delaying of emergence.
Introduction
Sevoflurane is widely used in pediatric anesthesia because of a fast and well tolerated inhaled induction, low cardiodepressive effect and hepatotoxicity, hemodynamic stability and rapid recovery from anesthesia. However, the occurrence of EA in children after sevoflurane anesthesia is common, with a reported incidence up to 80% [Citation1]. Although EA is a self-limited adverse event, it can cause injury to the child or to the surgical site and may also lead to the accidental removal of surgical dressings, intravenous catheters and drains; and extra nursing care may be necessary. So, several strategies should be carried out to prevent this distressing scenario. Pharmacological prevention of EA relies on analgesic and/or sedative agents.
Dexmedetomidine is a more highly specific α2-adrenoceptor agonist (α2/α1 = 1620/1) than clonidine (α2/α1 = 220/1), and has sedative and analgesic properties without significant respiratory depression at clinical dosages [Citation2]. Dexmedetomidine is reported to significantly reduce EA frequency after sevoflurane anesthesia in pediatric surgery and non-surgical procedures in inpatient and outpatient settings [Citation3,Citation4].
Propofol is a non-opioid, non-barbiturate, sedative-hypnotic agent with rapid onset and short duration of action [Citation5]. It showed an overall protective effect against EA, when given as continuous administration or as a bolus dose at the end of anesthesia [Citation6]. Ketamine is a phencyclidine derivative, classified as a dissociative sedative that provides analgesia and amnesia. It was also found that ketamine administration is effective in preventing EA [Citation7]. However, it is associated with significant adverse effects, including frightening emergence reactions, sympathomimetic effects and vomiting when administered in sedating doses. Combining low-dose ketamine with propofol (ketofol) has been used extensively as a part of procedural sedation and analgesia in the emergency department. The complementary effects of this combination are supposed to produce lower toxicity compared to each drug alone through decreasing required doses [Citation8]. We hypothesized that ketofol can be as effective as dexmedetomidine in prevention of EA due to its combined sedative and analgesic properties. So, we designed this study to compare the efficacy of ketofol and dexmedetomidine in reducing the incidence of EA in children.
Patients and methods
This study was carried out in El-Minia University Hospital during the period from April 2014 to March 2015 after institutional ethics committee approval and informed consents obtained from all patients’ parents prior to entry into the study. It involved 90 children of both sexes, aged 3–6 years old, ASA I or II scheduled to undergo orthopedic surgery under sevoflurane anesthesia. Patients with known allergy to any of the drugs used, with developmental delay, psychological, or neurological disorders, or with a history of chronic or acute intake of any sedative and analgesic drugs were excluded.
A careful medical history was taken from the parents. Then, general examination including (Heart rate, Blood Pressure, Respiratory rate) and physical examination including (chest, heart and abdomen) were done and complete blood picture was checked pre-operatively. Children were randomly allocated into 3 equal groups (30 children each) by means of random numbers generated by a computer:
Group K: received ketofol (ketamine 0.25 mg/kg and propofol 1.0 mg/kg in combination diluted to a volume of 10 ml by addition of normal saline), 10 min before the end of surgery.
Group D: received dexmedetomidine (0.3 mcg/kg diluted in normal saline to a volume of 10 ml), 10 min before the end of surgery.
Group C: received normal saline (10 ml), 10 min before the end of surgery (control group).
Children were fasted for 6 h without solid food and for 3 h without clear liquids and no premedication was given. Upon arrival into the operating room, induction of anesthesia started by inhalation of sevoflurane 8% in 100% O2 which was maintained till loss of consciousness. During induction of anesthesia, an electrocardiogram, pulse oximeter and noninvasive ABP monitor were attached.
After obtaining a sufficient depth of anesthesia, a peripheral intravenous line was inserted and fentanyl 2 μg/kg and atracurium 0.5 mg/kg were administered to facilitate endotracheal intubation. Direct laryngoscopy was performed and the trachea was intubated with an appropriate sized tube. Anesthesia was maintained with 100% O2 and sevoflurane 2–2.5 vol.% while mechanical ventilation was performed to sustain end tidal CO2 at 30–35 mmHg. Intraoperatively, HR, MAP, and SPO2 were recorded at the following measurement times i.e. preinduction, at induction, preintubation, postintubation, 5 min postintubation, every 10 min during the surgery, postextubation and then every 10 min for half an hour. Increase in HR and/or MAP >20% than baseline values was treated with incremental doses of fentanyl 1–2 mcg/kg.
Ten minutes before the completion of the procedure, the study drugs were administered to the patients by an anesthetist not involved in the study. Children in group K were given ketofol (ketamine 0.25 mg/kg and propofol 1.0 mg/kg in combination in 10 ml saline); those in group D were given dexmedetomidine (0.3 mcg/kg in 10 ml saline) while those in group C received normal saline (10 ml). After closure, sevoflurane anesthesia was discontinued and manual ventilation was performed with 100% O2. Residual muscle relaxation was reversed using neostigmine 0.05 mg/kg and atropine 0.02 mg/kg. The endotracheal tube was removed when patient’s gag reflex was restored, and regular spontaneous breathing was achieved. The time from sevoflurane discontinuation to the removal of ETT was recorded and defined as the extubation time. The duration of surgery and duration of sevoflurane exposure (from mask induction to the discontinuation of the inhaled anesthetic) were also recorded. Then children were transferred to the post-anesthesia care unit (PACU) and one of their parents accompanied them at the PACU until discharge.
In PACU, the incidence of EA was assessed using AONO’s 4-point scale [Citation9]. AONO’s scale was graded as follows: 1 = calm; 2 = not calm but could be easily consoled; 3 = moderately agitated or restless and not easily calmed; and 4 = combative, excited, or disoriented, thrashing around. Scores of one and two were considered as the absence of EA, and scores of three and four were analyzed as the presence of EA. The severity of EA was evaluated using pediatric anesthesia emergence delirium (PAED) scale devised by Sikich and Lerman [Citation10] (), a five-point rating scale with five grades for each item. The incidence and severity of EA were recorded upon awakening i.e. when the child had the first response to command or eye opening on command (T0), and every 10 min thereafter during the first 30 min (T10, T20 and T30). In case of agitation in the PACU, the first action is to encourage parental contact and when this failed midazolam 0.05–0.1 mg/kg was administered intravenously.
able 1 Pediatric anesthesia emergence delirium (PAED) scale.
Objective pain scale [Citation11] () was evaluated every 10 min. When patients had OPS ⩾5, they were given supplemental analgesics (paracetamol 15 mg/kg and/or diclofenac 1 mg/kg) and time from anesthetic discontinuation till 1st analgesic requirement was recorded.
able 2 Objective Pain Scale (OPS).
During PACU stay, MAP, HR and SpO2 were continuously monitored. MAP and HR were recorded upon arrival to the PACU, every 10 min and on PACU discharge. If oxygen saturation fell below 90%, oxygen face mask was given to the child.
Modified Aldrete score [Citation12] () was evaluated and adopted as the discharge criteria according to which a score ⩾9 is needed for discharge from PACU. The time from anesthetic discontinuation to attainment of this score was recorded. Children were discharged from the PACU to the ward when the required score was achieved without agitation, pain or vomiting.
able 3 Modified Aldrete scoring system.
.1 Statistical analysis
Data entry and analysis were all done with I.B.M. compatible computer using software called SPSS for windows version 11. Graphics was done using Excel. Quantitative data were presented by mean and standard deviation and qualitative data were presented as frequency distribution.
.1.1 Significance tests
| #x2013; | Chi square test: for categorical data. | ||||
| #x2013; | Kruskal wallis test: for nonparametric quantitative data. | ||||
| #x2013; | One way ANOVA test: for parametric quantitative data inside the group. | ||||
Based on the previously published studies [Citation3,Citation13], the sample size was calculated to detect 40% difference in the incidence of agitation between study and control groups with power of 0.80 a significance level of 0.05. Calculating for a 10% drop-out rate, 30 patients in each group were appropriate to detect this difference with any degree of confidence.
Results
This prospective, randomized, and double-blinded study included 90 pediatric patients, undergoing orthopedic surgery under sevoflurane anesthesia. Patients were randomized into 3 equal groups (30 patients each, K, D, and C groups).
There were no significant differences in age, weight, sex distribution, ASA physical status, duration of surgery, or duration of sevoflurane administration among the 3 groups ().
able 4 Demographic and anesthetic data of the study groups.
The incidence of EA in group K and group D was similar and significantly lower than that in group C at T0, T10, and T20. At 30 min after emergence, none of the patients had EA. Upon awakening, EA occurred in 26%, 16% and 90% of patients in groups K, D and C respectively. Over time, the incidence of EA decreased to be 10% in ketofol and dexmedetomidine groups and 60% in control group at T10. At T20, none of the patients in groups k and D developed EA while it occurred in 16.7% in control group ().
able 5 Incidence of emergence agitation at T0, T10, T20 and T30 in the study groups.
Regarding the severity of EA, the mean values of PAED score in group K (10.1 ± 0.35, 9.3 ± 1.15, 7 ± 0) and in group D (10 ± 0.25, 9.1 ± 0.57, and 6.9 ± 0.57) at T0, T10, T20 respectively were significantly lower than the corresponding values in control group (15.2 ± 0.8, 12 ± 1.3, 9 ± 0) (). There was no significant differences between ketofol and dexmedetomidine group at any time during assessment.
able 6 PAED score (Mean ± SD) at T0, T10, T20 and T30 of the study groups.
Time to extubation was significantly longer in dexmedetomidine group than that in ketofol group (12.8 ± 1.95 min. in group D versus 9.08 ± 1.7 min. in group K, p < 0.001). When compared to control group, both of study drugs (Ketofol and dexmedetomidine), showed significantly longer extubation times. (). Patients in dexmedetomidine group had more sedation score as shown by the longer time to get modified Alderete score ⩾9 (17.1 ± 2.5 min.) compared to ketofol (p < 0.001) and control (p < 0.001). Ketofol group took longer time to get modified Alderet score ⩾9 than that of control group. (). Ketofol provided more effective analgesia in the early postoperative period than dexmedetomidine where time of 1st analgesic requirement was significantly longer in group K than that of group D (91.58 ± 12.2 min in group K versus 34.1 ± 6 min in group D, p < 0.001). Both ketofol and dexmedetomidine provided more prolonged analgesia when compared to control group (9.2 ± 2.4 min in group C, p < 0.001) ().
able 7 Recovery characteristics of the study groups.
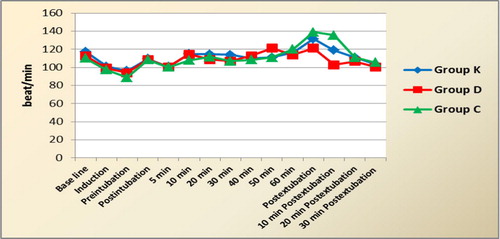
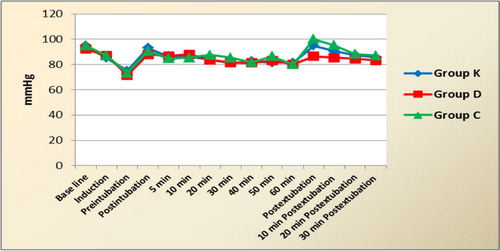
As regard heart rate and mean arterial pressure (MAP), they decreased after induction in all groups with no significant differences among them or significant differences when compared to baseline values. Also, there was no significant differences between the readings among the 3 groups throughout the surgery till extubation time. After tracheal extubation, the pressor response of extubation was more evident in control group, where HR and MAP were significantly higher than the corresponding values in ketofol and dexmedetomidine groups at postextubation and 10 min later. Also, the hemodynamic variables recorded postextubation and 10 min later were significantly higher in group K than group D. Within each group, HR and MAP values did not change significantly from baseline ones at any time during the study ( and ). Concerning SpO2, there was no significant changes in the readings among the 3 groups or when compared to baseline values within each group throughout the surgery and recovery period.
Discussion
EA is a troublesome clinical phenomenon of uncertain etiology. The incidence of EA varies from 2% to 80% [Citation6] and depends on the hypnotic agent used {incidence increases with the new fast-acting and less soluble volatile agents named sevoflurane and desflurane compared to halothane or isoflurane [Citation14].
Prevention of EA depends mainly on reducing preoperative anxiety, removing postoperative pain and administration of sedative and/or analgesic agents. Many studies focused on the pharmacologic preventive strategies against emergence agitation and found several drugs efficient in the prevention of this adverse event. These preventive treatments included propofol given at the end of surgery [Citation6] or by continuous administration during surgery [Citation15]; intraoperative fentanyl [Citation6]; ketamine [Citation16]; clonidine [Citation17]; dexmedetomidine [Citation3,Citation18,Citation19]; preoperative gabapentin[Citation20]; midazolam [Citation17]; intraoperative magnesium infusion [Citation21]; preoperative midazolam [Citation22] and intraoperative dexamethasone [Citation23].
The researches in the field of emergence agitation usually compare these preventive treatments to find the most efficient agents in this indication with minimal side effects.
To our knowledge, this is the first study comparing the effects of dexmedetomidine and ketofol in prevention of EA in children under sevoflurane anesthesia. We found that ketofol was as effective as dexmedetomidine in preventing EA where incidence of ED in both groups (K and D) was similar and significantly lower than that in control group. Ketofol provided better analgesic effect with earlier recovery, when compared to control and dexmedetomidine groups.
Dexmedetomidine, a highly selective alpha 2 adrenoceptor agonist, is of great interest in prevention of EA due to its sedative and analgesic effects. Several studies proved its efficacy in reducing the incidence of postanesthesia agitation in children by 57–70% compared with control groups [Citation3,Citation24–Citation28]. Consistent with Guler’s study [Citation24], dexmedetomidine group showed longer time to extubation and discharge from PACU than in ketofol and control groups. Other studies did not demonstrate prolongation of emergence or discharge time [Citation3,Citation25,Citation26]. This difference may be due to variable dosing, route or timing of administration of dexmedetomidine.
Regarding the effectiveness of propofol in prevention of EA, propofol given as an induction bolus showed no efficiency in decreasing ED occurrence [Citation6]. This is probably related to its short half-life way out which makes its blood and effect site concentration below its therapeutic effect at recovery from anesthesia. Several studies have suggested that a single administration of 1 mg/kg of propofol at the discontinuation of anesthesia is effective in reducing EA without delay of discharge from the PACU in children receiving sevoflurane for induction and maintenance of anesthesia. The decreased incidence of EA could be accounted for the residual sedative effect and euphoric effect of propofol in the early recovery period [Citation29].
Aouad et al. reported that the administration of 1 mg/kg propofol at the end of surgery in children, undergoing strabismus surgery, significantly decreased the incidence of EA (19.5% compared to 47.2% in the placebo group). Recovery time was increased in the propofol group compared to the placebo group [Citation30]. In our study, ketofol group also showed longer extubation time and delayed discharge from PACU when compared to control group. Abu-Shahwan’s study showed that the administration of subhypnotic doses of propofol (1 mg/kg) at the end of sevoflurane general anesthesia was effective in decreasing the incidence and severity of EA in children undergoing MRI (4.8% incidence of EA in propofol group compared to 26.8% in the placebo group [Citation31]. Compared to the Aouad study, this better outcome can be attributed to setting the PAED acceptable score at 16 and the nonpainful nature of MRI. On the other hand, another study found that administration of propofol 1 mg/kg at the end of surgery did not have any significant effect in reducing the incidence and severity of EA in children (aged 3–8 years), undergoing adenotonsillectomy under sevoflurane anesthesia [Citation32]. They explained failure of propofol to prevent EA in their research because the surgery in their study (adenotonsillectomy) was different from that in Aouad‘s (strabismus surgery). Although the surgery type itself that involves the tonsil, middle ear, and eye is a major factor for inducing EA, adenotonsillectomy is the more important independent risk factor for EA than strabismus surgery [Citation33]. Eckenhoff et al. speculated that sense of suffocation during emergence from anesthesia may contribute to EA in patients undergoing head and neck surgery [Citation34].
Ketamine has been used successfully to reduce the incidence and severity of EA, when given in small doses (0.25 mg/kg) at the end of MRI procedures and dental repair surgery under sevoflurane anesthesia [Citation35,Citation36]. Lee and colleagues confirmed the effectiveness of small doses of ketamine (0.25 and 0.5 mg/kg), given 10 min. before the end of surgery in decreasing the incidence of EA compared to control group in children, undergoing adenotonsillectomy [Citation16]. There were no significant differences in extubation time, time to discharge from PACU or PONV between the three groups but the K 0.5 group showed a lower pain score than K 0.25 group. In our study, we chose the smaller dose of ketamine (0.25 mg/kg) in combination with propofol (ketofol) to avoid excessive delay in emergence or PACU discharge time. The added effects of ketamine and propofol prolonged extubation and PACU discharge time compared to the control group.
In comparison with ketamine, dexmedetomidine was comparable in reducing the incidence of EA and pain after sevoflurane anesthesia for pediatric strabismus surgery [Citation19]. Incidence of POV was lower in dexmedetomidine group (15%) than in Ketamine (44%) or placebo (45.8%) groups. Awakening time and time to discharge from PACU were longer in dexmedetomidine and ketamine groups than in placebo group. On the contrary, another trial failed to show any beneficial effects of ketamine (0.25 mg/kg) or midazolam (0.03 mg/kg), given before end of surgery, for the prevention of EA in children undergoing abdominal and genital surgery, compared to placebo [Citation37]. The authors explained that parental presence in the PACU and good pain relief with caudal block resulted in satisfactory PAED scores (⩽10) in the 3 groups.
Ketofol (ketamine in combination with propofol) has been used for procedural sedation in children and it was found that low-dose ketamine effectively offsets the cardiorespiratory depression caused by propofol while providing adequate sedation and analgesia [Citation38]. Ketamine in low-dose has been used successfully to prevent recurrence of EA in subsequent anesthetic sessions in seven children, anesthetized with prolonged propofol total intravenous anesthesia for radiation therapy [Citation39]. Another study showed that ketofol was as effective as propofol in the prevention of EA in children undergoing adenoidectomy or adenotonsillectomy under sevoflurane anesthesia. In addition, ketofol had superior analgesic effect demonstrated by lower OPS on arrival to PACU and 10 and 20 min. later, when compared to propofol and control groups [Citation40]. Similarly, our data proved the comparable efficacy of ketofol to dexmedetomidine in preventing EA. However, it had better analgesic effect, when compared to dexmedetomidine and control groups.
Concerning the hemodynamic effects of the study drugs, MAP and HR decreased after induction in all groups but this reduction was clinically acceptable (±20% of baseline values) and did not need any pharmacological intervention. The only significant changes in hemodynamics occurred postextubation, where MAP and HR were significantly lower in ketofol and dexmedetomidine groups than those of control group postextubation and 10 min. later. This could be attributed to the exaggerated pressor response in the control group and higher incidence and severity of EA in the early recovery period in control group compared to ketofol and dexmedetomidine groups.
Throughout the study, hemodynamics within each group did not change significantly compared to baseline values at any time either after administration of the study drugs or after extubation. This may be due to use of small doses of the study drugs.
In children, large doses of dexmedetomidine cause peripheral vasoconstriction, which may lead to transient systemic hypertension whereas low doses cause central sympatholysis, which can lead to systemic hypotension. In healthy children, the severity of hypotension varies directly with the dose of dexmedetomidine. When a loading dose between 0.5 and 1 μg/kg dexmedetomidine is administered over 10 min as the sole sedative, systolic BP decreases as the dose increases, reaching a maximum decrease of 30% from baseline at 1 μg/kg [Citation41]. When a small loading dose of 0.5 μg/kg dexmedetomidine is infused over 5 min during 1 minimum alveolar concentration sevoflurane and desflurane, systolic BP decreases only 10% [Citation2]. In our study, we used 0.3 μg/kg dexmedetomidine for the prevention of EA. This small dose seemed to maintain the hemodynamic stability throughout the study. Similarly, addition of low-dose ketamine to propofol preserved MAP without prolonging recovery or increasing the incidence of adverse events in children, undergoing cardiac catheterization, lumbar puncture and bone marrow aspiration [Citation38,Citation42].
Conflict of interest
The authors declared that there is no conflict of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- Z.Yan-linX.Hong-boThe clinical research to sevoflurane emergence agitation in childrenJ Clin Anesthesiol262010988992
- E.DeutschJ.D.TobiasHemodynamic and respiratory changes following dexmedetomidine administration during general anaesthesia: sevoflurane vs desfluranePaediatr. Anaesth.172007438444
- M.E.IbacacheH.R.MuñozV.BrandesA.L.MoralesSingle-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in childrenAnesth.-Analg.98120046063
- B.IkM.ArslanA.D.TungaO.KurtipekDexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgeryPaediatr. Anaesth.162006748753
- E.L.BahnK.R.HoltProcedural sedation and analgesia: a review and new conceptsEmergency Med Clin North Am.232005503517
- S.DahmaniI.StanyC.BrasherPharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studiesBr. J. Anaesth.10422010216223
- S.M.GreenB.KraussThe semantics of ketamine [editorial]Ann Emergency Med392000480482
- S.AroraCombining ketamine and propofol (“ketofol”) for emergency department procedural sedation and analgesia: a reviewWestern J Emergency Med920082023
- J.AonoW.UedaK.MamiyaE.TakimotoM.ManabeGreater incidence of delirium during recovery from sevoflurane anesthesia in preschool boysAnesthesiology87199712981300
- N.SikichJ.LermanDevelopment and psychometric evaluation of the pediatric anesthesia emergence delirium scaleAnesthesiology100200411381145
- R.HannallahL.BroadmanComparison of caudal and ilioinguinal/iliohypogastric nerve blocks for control of post-orchidopexy pain in pediatric ambulatory surgeryAnaesthesiology661987832834
- J.A.AldreteThe post-anesthesia recovery score revisitedJ Clin Anesth7119958991
- M.A.Abdel-Ma’boudEffect of dexemeditomedine and propofol on the prevention of emergence agitation following sevoflurane anesthesia in Egyptian childrenJ Egypt Soc Parasitol4432014687694
- N.KurataniY.OiGreater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trialsAnesthesiology1092008225232
- A.KanayaN.KurataniD.SatohS.KurosawaLower incidence of emergence agitation in children after propofol anesthesia compared with sevoflurane: a meta-analysis of randomized controlled trialsJ Anesth282013411
- Y.S.LeeW.Y.KimJ.H.ChoiJ.H.SonJ.H.KimY.C.ParkThe effect of ketamine on the incidence of emergence agitation in children undergoing tonsillectomy and adenoidectomy under sevoflurane general anesthesiaKorean J Anesthesiol5852010440445
- C.ZhangJ.LiD.ZhaoY.WangProphylactic midazolam and clonidine for emergence agitation in children after emergence from sevoflurane anesthesia: a meta-analysisClin Ther35201316221631
- M.A.AliA.A.AbdellatifPrevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofolSaudi J Anaesth72013296300
- J.-Y.ChenJ.-E.JiaT.-J.LiuComparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in childrenCan J Anaesth602013385392
- A.E.SalmanA.CamkıranS.OğuzA.DönmezGabapentin premedication for postoperative analgesia and emergence agitation after sevoflurane anesthesia in pediatric patientsAgri252013163168
- M.AbdulatifA.AhmedA.MukhtarS.BadawyThe effect of magnesium sulphate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anaesthesiaAnaesthesia68201310451052
- O.KonerH.TureA.MercanEffects of hydroxyzine-midazolam premedication on sevoflurane-induced paediatric emergence agitation: a prospective randomised clinical trialEur J Anaesthesiol282011640645
- G.KhaliliP.SajediA.ShafaA randomized evaluation of intravenous dexamethasone versus oral acetaminophen codeine in pediatric adenotonsillectomy: emergence agitation andanalgesiaMiddle East J Anesthesiol212012499504
- G.GulerA.AkinZ.TosunS.OrsA.EsmaogluA.BoyaciSingle-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomyPaediatr. Anaesth.1592005 Sep762766
- M.ShukryM.C.ClydeP.L.KalarickalU.RamadhyaniDoes dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia?Paediatr Anaesth15200510981104
- B.IsikM.ArslanA.D.TungaDexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgeryPaediatr Anaesth162006748753
- A.PatelM.DavidsonM.C.TranDexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomyAnesth Analg111201010041010
- L.SunR.GuoL.SunDexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trialsActa Anaesthesiol Scand5862014 Jul642650
- S.NakayamaH.FurukawaH.YanaiPropofol reduces the incidence of emergence agitation in preschool-aged children as well as in school-aged children: a comparison with sevofluraneJ Anesth2120071923
- M.T.AouadV.G.Yazbeck-KaramV.G.NasrM.F.El-KhatibG.E.KanaziJ.H.BleikA single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesiaAnesthesiology1072007733738
- I.Abu-ShahwanEffect of propofol on emergence behavior in children after sevoflurane general anesthesiaPaediatr Anaesth1820085559
- C.J.LeeS.E.LeeM.K.OhThe effect of propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomyKorean J Anesthesiol59220107581
- T.Voepel-LewisS.MalviyaA.R.TaitA prospective cohort study of emergence agitation in the pediatric postanesthesia care unitAnesth Analg96200316251630
- J.E.EckenhoffD.H.KnealeR.D.DrippsThe incidence and etiology of postanesthetic excitement. A clinical surveyAnesthesiology221961667673
- B.J.DalensA.M.PinardD.R.LetourneaPrevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesiaAnesth Analg102200610561061
- I.Abu-ShahwanK.ChowdaryKetamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesiaPediatr. Anesth.172007846850
- A.OzcanA.G.KayaN.OzcanG.M.KaraaslanE.ErB.BaltaciH.BasarEffects of ketamine and midazolam on emergence agitation after sevoflurane anaesthesia in children receiving caudal block: a randomized trialBraz J Anesthesiol6462014377381
- A.AkinA.EsmaogluG.GulerR.DemirciogluN.NarinA.BoyaciPropofol and propofol-ketamine in pediatric patients undergoing cardiac catheterizationPediatr Cardiol2652005553557
- D.L.AnghelescuL.C.RakesJ.R.ShearerG.B.BikhaziPrevention of emergence agitation in seven children receiving low-dose ketamine and propofol total intravenous anesthesiaAANA7932011 June238242
- S.N.RizkE.M.SamirUse of ketofol to control emergence agitation in children undergoing adenotonsillectomyEgypt J Anaesthesia30120141319
- G.C.PetrozN.SikichM.JamesH.van DykS.L.ShaferM.SchilyJ.LermanA phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in childrenAnesthesiology105200610981110
- A.G.YazdiV.AyatollahiA.HashemiEffect of two different concentrations of propofol and ketamine combinations (ketofol) in pediatric patients under lumbar puncture or bone marrow aspirationIran J Ped Hematol Oncol312013187192