Abstract
Background
This study evaluated the efficacy of transdermal nicotine (TDN) delivery system (15 mg/16 h) or transdermal melatonin (TDM) delivery system (7 mg) 2 h preoperatively for acute postoperative pain after laparoscopic cholecystectomy compared to placebo group (C).
Methods
Sixty female non-smoker patients, aged 18–50 years and ASA I and II undergoing elective laparoscopic cholecystectomy under general anesthesia were included in this randomized controlled double-blind study. Patients were randomly divided into 3 groups 20 each, and C group patients received transdermal placebo patch, TDN group (15 mg/16 h) and TDM group (7 mg/8 h). Assessment of postoperative pain, sedation, hemodynamic variables such as HR and MAP, postoperative monitoring of arterial SpO2 and side effects (e.g. nausea, vomiting, pruritus, respiratory depression and hemodynamic instability) were done 30 min, 1, 2, 6 and 12 h postoperatively. Postoperative Patient’s and Surgeons’ satisfaction, Intraoperative bleeding and plasma cortisol (μg/dl) 2 h postoperatively were also assessed.
Results
There was a significant reduction in the VAS score, total pethidine requirements (mg) and significantly higher patient’s satisfaction in TDN and TDM groups when compared with the C group postoperatively. The sedation score and surgeons’ satisfaction were significantly higher associated with a significant decrease in MAP and Intraoperative bleeding in TDM group compared to C and TDN groups postoperatively. Significant nausea and vomiting in TDN group and significant sedation in TDM group were recorded.
Conclusion
The use of preoperative TDN (15 mg/16 h) or TDM (7 mg/8 h) was an effective and a safe adjuvant for acute pain after surgery.
Introduction
Intraoperative and postoperative noxious inputs may cause central sensitization, but analgesic interventions given before the noxious stimulus may attenuate or block sensitization [Citation1]. Preventing the establishment of altered central processing by analgesic treatment may result in short-term (e.g., reduction in postoperative pain and accelerated recovery) and long-term (e.g., reduction in chronic pain and improvement in health related quality of life) benefits during a patient’s convalescence [Citation2]. Early postoperative pain is the most common complaint after elective laparoscopic cholecystectomy. In 17–41% of the patients, pain is the main reason for overnight hospital stay after day case surgery [Citation3]. Intense acute pain after laparoscopic cholecystectomy might predict the development of chronic pain (e.g. post-laparoscopic cholecystectomy syndrome) [Citation4]. These concepts suggested a possible study design; effective analgesia starts before incision and covers both the period of surgery and the postoperative period.
Transdermal drug delivery offers the potential benefits of simplicity, efficacy and patient acceptance. In theory, a transdermal delivery system can provide a stable serum concentration for an extended period of time with acceptable interpatients’ variability [Citation5].
Nicotine, a potent stimulant found in cigarette smoke, was found to have analgesic properties [Citation6]. Nicotine acts on nicotinic cholinergic receptors, which are found in the central nervous system, autonomic ganglia, the neuromuscular junction, as well as in several non-neuronal tissues [Citation7]. Nicotine reduced nociceptive input to the superficial and deep dorsal horn and provides support for α4β2 and α7 nicotinic-mediated antinociceptive actions [Citation8]. Nicotine acts on nAChRs in both the brain and the spinal cord to activate spinal cord descending inhibitory pain pathways [Citation7]. Nicotine-mediated analgesia is thought to involve, at least in part, local release of norepinephrine, with activation of α2-adrenergic receptors [Citation9]. Morphine activates a descending inhibitory system, leading to increased release of endogenous acetylcholine in the spinal cord, thereby producing analgesia through activation of spinal muscarinic and nicotinic receptors [Citation10]. Nicotine may also produce analgesia by release of endogenous opioids [Citation11]. Furthermore, nicotine has anti-inflammatory actions that could reduce pain [Citation12,Citation13]. Smokers would not experience pain relief from nicotine because of chronic exposure to nicotine and receptor inactivation [Citation14]. Only full-strength nicotine patches (15 mg/16 h or 21 mg/24 h) are subsidized on the Pharmaceutical Benefits Scheme (PBS) [Citation15].
Melatonin (N-acetyl-5-methoxytryptamine, MT) is a hormone secreted primarily by the pineal gland in a circadian fashion. The synthesis and secretion of MT are induced by darkness and suppressed by light through retinal nerve fibers projecting to the suprachiasmatic nucleus of hypothalamus, then to the superior cervical ganglion and finally to the pineal gland. During the night, the mean endogenous plasma concentration of MT is ∼50–70 pg/mL (216–302 pmol/L) in young adults. In daylight hours, the mean MT plasma concentration is typically <10 pg/mL (43 pmol/L). Plasma MT levels begin to increase at ∼2100 h, peak between 0200 and 0400 h, and return to baseline at 0700–0900 h [Citation16]. MT has a short plasma elimination half-life, ∼45 min and when administered orally, shows low and variable bioavailability due to extensive first-pass metabolism and/or variable absorption [Citation17]. Transdermal delivery system for melatonin (TDM) results in sustained plasma MT levels that can be tailored to the normal physiological range and avoid the first-pass metabolism. TDM is intended to be worn for 8 h [Citation18]. Melatonin has sedative, anxiolytic, analgesic, antihypertensive, anti‐inflammatory, chronobiotic and oncostatic effects and potent antioxidant properties [Citation19]. Melatonin exerts its analgesic effects through augmentation of GABA−ergic systems and morphine anti-nociception, enhancing GABA−induced currents and inhibiting glycine effects [Citation20]. Melatonin may enhance the levels of β−endorphins and the anti-nociception induced by delta opioid receptor agonists and could activate MT2 melatonin receptors in the dorsal horn of the spinal cord [Citation21,Citation22]. The long-lasting analgesia induced by melatonin can be blocked by naloxone suggests that opioid receptors are involved in actions of melatonin [Citation23].
The stress response to surgery is characterized by increased secretion of pituitary hormones and activation of the sympathetic nervous system. The changes in the pituitary secretions have secondary effects on hormone secretion from target organs (increased secretion of cortisol from the adrenal cortex) [Citation24].
In this randomized, double-blind, placebo-controlled study, our primary objective was to compare the TDN patches to TDM patches for relieving postoperative pain after laparoscopic cholecystectomy under general anesthesia to detect a mean difference of total analgesic (pethidine) consumption. And our secondary goal was to compare the effects of TDN patches to TDM patches on prolongation of first analgesic requirement time, pain score, sedation score, stress response, patient satisfactory score, surgeon satisfactory score, postoperative monitoring of heart rate, mean arterial blood pressure, arterial SpO2 and side effects (e.g. nausea, vomiting, pruritus, respiratory depression and hemodynamic instability).
Methods
This study was designed to be a randomized, placebo-controlled, double-blind parallel study in which the patients, investigators, anesthesiologists and the surgeons were blinded to the given treatment. This study was conducted in Ain-Shams university hospitals, from April 2013 to July 2015, on 60 female non-smoker patients aged between 18 and 50 years old of ASA physical status I and II of 70–90 kg body weight and height 160–180 cm undergoing elective laparoscopic cholecystectomy under general anesthesia. The study protocol was approved from the institutional ethical committee and written informed consent was obtained from all the patients (see ).
able 1 Ramsay sedation score.
Patients with impaired kidney or liver functions, history of cardiac or central nervous system disease, history of smoking, history of drug or alcohol abuse, history of chronic pain or daily intake of analgesics, uncontrolled medical disease (diabetes mellitus and hypertension), history of intake of non-steroidal anti-inflammatory drugs or opioids within 24 h before surgery or allergy to the used medications, coagulation defect, local infection at the site of application of transdermal patch, patient refusal or duration of surgery more than 120 min were excluded from the study.
Patients were randomly divided into 3 groups, C group (n = 20) each patient received transdermal placebo patch, TDN group (n = 20) each patient received transdermal therapeutic system-nicotine (15 mg/16 h) and TDM group (n = 20) each patient received transdermal therapeutic system-melatonin (7 mg/8 h). Randomization was done using computer-generated number table of random numbers in a 1:1 ratio and conducted using sequentially numbered, opaque and sealed envelope (SNOSE). All patches were placed 2 h preoperatively and were applied to the skin in the subclavicular area and the area was not shaved to maintain the integrity of the skin to maintain normal absorption (if necessary hair was only clipped from the patch site prior to application). The patch was removed 12 h post-operatively. In this study we used identical placebo patches assembled by the hospital pharmacy, we used Nicorette® invisi 15 mg patch releasing 15 mg of nicotine over 16 h, produced by Lohmann Therapie-System, Germany, and we used melatonin sleep patch from Respro Labs™ containing 7 mg of melatonin. Active patches containing nicotine or melatonin were indistinguishable from placebo patches, covered with adhesive plaster to confirm fixation and to blind the anesthetists and observers for the type of the used patches. The study drugs were prepared by the anesthesia resident not involved in any other part of the study.
Before any patch placement a preoperative visit was done to all patients to assess patient fitness for operation, to alleviate anxiety, to inform them about transdermal patches (its efficacy in the treatment of postoperative pain, its possible side effects and method of application) and to make them familiarized with 10 cm marked visual analogue scale (VAS) for postoperative assessment of pain, where 0 cm defines no pain and 10 cm defines the maximum intolerable pain. The patients were also assured that they would receive intramuscular injection (IM) of pethidine 0.5 mg/kg once they start to first experienced pain postoperatively (Patients with (VAS > 3). Time to the first request for analgesic and the total pethidine consumption (mg) in 12 h postoperatively was recorded.
The general anesthesia technique was standardized for all the patients as well as monitors including 5 lead ECG, non-invasive blood pressure (NIBP) monitor, pulse oximetry and capnography after intubation using Datascope monitors. Neuromuscular function was also monitored using a peripheral nerve stimulator. After establishing an intravenous (IV) line, induction of general anesthesia with fentanyl (2 ug/kg) and sleeping dose of propofol followed by rocuronium (0.6 mg/kg) to facilitate orotracheal intubation was done. Anesthesia was maintained using sevoflurane in oxygen and air. Granisetron (1 mg IV) was given as a prophylactic antiemetic. At the end of the surgery, the residual neuromuscular paralysis was antagonized with neostigmine (0.05 mg/kg) and atropine (0.01 mg/kg). After satisfactory recovery, patients were extubated and transferred to the post-anesthesia care unit (PACU) where they were monitored with ECG, NIBP and pulse oximetry.
Assessment of postoperative pain, sedation, hemodynamic variables such as heart rate (HR) and mean arterial pressure (MAP), postoperative monitoring of arterial SpO2 and side effects (e.g. nausea, vomiting, pruritus, respiratory depression and hemodynamic instability) was done 30 min, 1, 2, 6 and 12 h postoperatively.
Postoperative pain was evaluated based on visual analogue scale, and first time to ask for rescue analgesia and total pethidine requirements (mg) in 12 h postoperatively was also recorded. Assessment of sedation was according to sedation score (Ramsay sedation score) [Citation25].
Hypotension was considered if there was 20% decrease below the baseline for mean arterial blood pressure, and it was treated with intravenous ephedrine (3–6 mg IV bolus). Bradycardia (heart rate <55 beats/min) was treated with intravenous atropine (0.6–1 mg). If there was a decrease in arterial SpO2 (<90%), it was treated with oxygen through a transparent face mask. Severe nausea or vomiting was treated with dexamethasone 5 mg and severe pruritus was treated with clemastine (TavegylR) 2 mg/ampoule i.v. as required. If any of the patients developed respiratory depression, the transdermal patch was removed and intermittent doses of naloxone 0.4 mg i.v. were administered.
Patient’s satisfaction was done by asking the patient to answer the question, ‘How would you rate your experience after the surgery?’ using a 7-point Likert verbal rating scale () [Citation26]. Surgeons were also asked to rate their satisfaction with operative conditions, using the 7-point Likert verbal rating scale at the end of surgery, acceptable satisfaction score of both the patient and surgeon being 5–7. Intraoperative bleeding was assessed by bleeding scale (0–5) () [Citation27], acceptable bleeding score being 0–2.
able 2 Intraoperative bleeding score classification.
Hormonal stress response was assessed through recording plasma cortisol (ug/dl) 2 h postoperatively. Serum cortisol was measured by a Fluorescence Polarization Immunoassay Technology (FPIA) by the Abbott AXSYM system with the following reference ranges (morning serum cortisol 4.2–38.4 ug/dl and evening serum cortisol 1.7–16.6 ug/dl).
.1 Analysis of data
Using PASS 11 for sample size calculation, in a one-way ANOVA study it was calculated that a sample size of 19 patients per group will achieve 82% power to detect a mean difference of 50 mg in total Pethidine consumption with a SD of 25 using an F test with a 0.05 significance level. 20 patients per group were intended to be included to replace any dropouts.
Data were analyzed using SPSS 21.0 for Windows (SPSS, Chicago, IL, USA). Analysis of variance was used to compare the three groups for quantitative parametric data with post hoc Tukey’s test performed if there was a significant difference among the groups, a Kruskal–wallis test was used for quantitative nonparametric data. Chi-square test was used for comparison of qualitative data. Continuous parametric data were presented as mean ± SD, nonparametric data as median (IQR) and categorical data were presented as number of patients. P-values of <0.05 were considered statistically significant.
Results
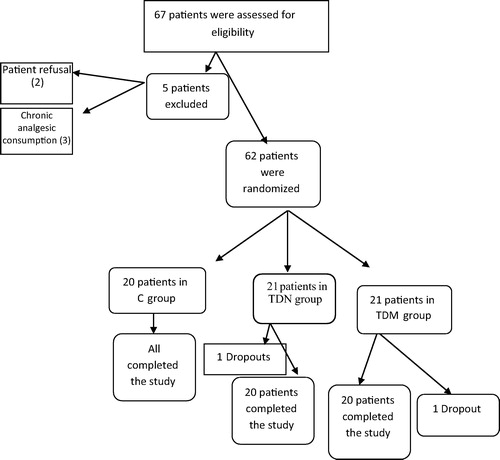
A total of 67 patients were assessed for eligibility from April 2013 to July 2015 (), out of which 62 patients received study medication after randomization, 60 patients completed the study (20 patients for each group) and their data were included in the final analysis (). Five patients were not included in this study on account of patient’s refusal (2 patients) and history of chronic analgesic consumption (3 patients). Two patients were considered as dropouts after initial randomization and were therefore not subjected to further statistical analysis (the two patients needed re-exploration on account of the postoperative bleed).
Results of the current study did not show significant difference in the demographic data of the groups of patients as regards age, body weight, height, ASA physical status and the length of surgery in minutes as shown in .
able 3 The demographic data.
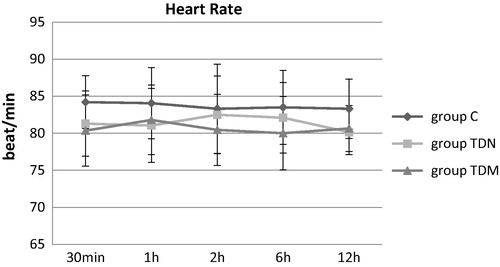
No significant differences in HR between the three groups were recorded at any time postoperatively as shown in . There were lower recorded values in the mean heart rate in the TDN and the TDM groups compared to C group, 30 min, 1 h, 2 h and 6 h postoperatively and this wasn’t significant. There was a decrease in the mean heart rate in the TDN group compared to the C and the TDM groups 12 h postoperatively and this wasn’t significant.
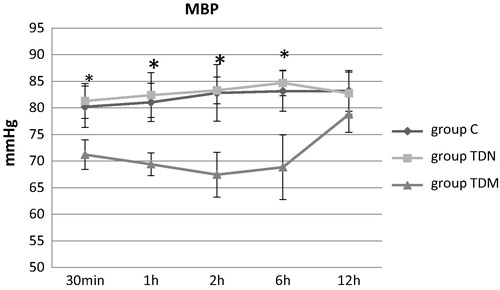
There was a significant decrease in the mean arterial blood pressure in the TDM group compared to the C and the TDN groups, 30 min, 1 h, 2 h and 6 h postoperatively as shown in .
No significant changes were noted in the SpO2 between the studied groups throughout the study period (P > 0.05).
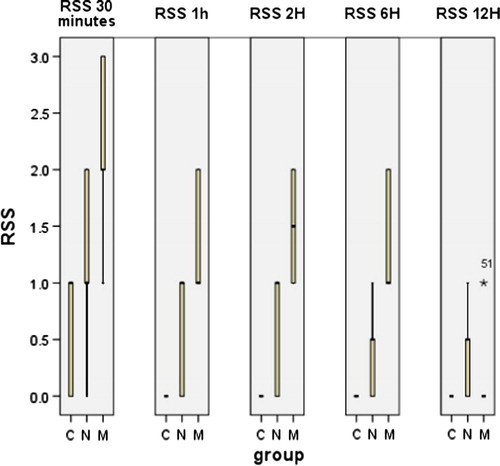
The sedation score was significantly higher in TDM group compared to the C and the TDN groups, 30 min, 1, 2, and 6 h postoperatively as shown in .
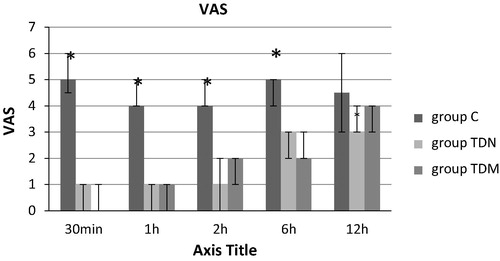
There were significantly lower recorded values in visual analogue scale in the TDN group and TDM group in comparison with C group, 30 min, 1, 2 and 6 h postoperatively. Pain score was lower in the TDN group compared to the C and the TDM groups, 12 h postoperatively and this was not significant as shown in .
As regards side effects in our study, all cases of the 3 groups were hemodynamically stable, no patient developed hypoxia and there were no reported intraoperative complications interfering with the course of surgery or interrupting the surgeons. Two patients in the C group suffered from nausea (p = 0.01). In the TDN group, the postoperative nausea and vomiting (PONV) occurred as follows: nausea (8 cases, p = 0.004) and vomiting (4 cases, p = 0.009), in spite of preoperative granisetron (1 mg IV) was given as a prophylactic antiemetic and there were no reported cases of erythema, respiratory depression or pruritus. In the TDM group, two patients were dizzy (p = 0.104).
There was significant difference postoperatively between the TDN and the TDM groups in comparison with C group as regards the time for 1st rescue analgesic (minutes) postop, the total pethidine requirements (mg) 12 h postop, the serum cortisol (μg/dl) 2 h postop and the patient’s satisfaction score. Intraoperative bleeding measured by bleeding scale was statistically significant less in the TDM group than in the C and the TDN groups. The surgeon’s satisfaction score was significantly higher in the TDM group compared to the C and the TDN groups as shown in .
able 4 Postoperative data.
Discussion
In the present study, there was an overall significant reduction in the VAS score in the TDN and TDM groups when compared with the C group in the 1st 6 h postoperatively. Even in the next 6 h where there was no significant reduction in the VAS, it was still lower in the TDN group than the C and TDM groups. This reduction in the VAS score was associated with significant delay in the postoperative time for 1st rescue analgesic (minutes), significant reduction in the postoperative total pethidine requirements (mg) 12 h postoperatively, significant reduction in the 2 h postoperative serum cortisol (μg/dl) and the significant increase in the postoperative patient’s satisfaction score as compared with the group C.
Also, this study revealed that the sedation score was significantly higher in group (III) compared to groups (I) and (II) 30 min, 1, 2, and 6 h postoperatively.
The choice of the transdermal delivery system of nicotine with a predicted delivery rate of 15 mg/16 h was based on a limitation mentioned by Habib et al. who stated that the preoperative application of a 7 mg nicotine patch resulted in a significant reduction in postoperative opioid consumption in nonsmoking men undergoing radical retropubic prostatectomy (RRP) under general anesthesia to establish whether there is a dose–response relationship for the analgesic effect of nicotine [Citation28].
The current study was based on a selective basis to equalize intragroup difference and to allow evaluation of the effect of the study drug. All patients were chosen to be female patients because of difference in pain thresholds between both genders, which was previously proved to be significantly higher in female patients who mostly consume more rescue analgesia than in male patients and was attributed to higher levels of apprehension [Citation29,Citation30]. In one study, a transdermal nicotine patch delivered 7 mg/24 h to nonsmokers and 21 mg/24 h to smokers, and nicotine increased the pain threshold and tolerance ratings of men but had no effect on the pain ratings of women [Citation31]. Current smokers were excluded from the study depending on the study by Olson et al. who indicated that transdermal nicotine, 5–15 mg, failed to relieve PO pain or reduce opioid use in smokers [Citation14]. In addition, Richardson et al. documented that the analgesic or enhanced nociceptive effect of nicotine may depend on tobacco use history [Citation32].
These results are in agreement with the findings of Habib et al. who found that patients underwent radical retropubic prostatectomy and were treated with preoperative TDN patch 7 mg showed significantly lower cumulative morphine consumption at 24 h [Citation28]. Our results were also supported with the study by Ahmed Nagy and ElKadi who demonstrated that using TDN patch (5 mg/16 h) as an analgesic modality adjunctive to thoracic epidural analgesia (TEA) for patients undergoing thoracotomy was accompanied by significantly lower VAS scores and significantly lower consumption of rescue analgesia in patients who received nicotine patch compared with those who received placebo [Citation33]. These results coincide with the study by Ali and Sakr who reported that pre-emptive application of transdermal 7 mg nicotine patch 2 h before surgery, had an analgesic effect in patients undergoing lumber disk surgery reducing the intra-operative fentanyl requirements and postoperative cumulative morphine consumptions and led to improved pain control [Citation34]. Yagoubian et al., documented that preoperatively administered nicotine nasal spray (3 mg) was associated with a highly significant decrease in pain reported during the 5 days after third molar surgery [Citation35].
In contrast to our results, the systematic review conducted by Mishriky and Habib documented that the perioperative nicotine administration was associated with a statistically insignificant reduction in pain scores at 24 h but with statistically significant reduction in cumulative opioid consumption at 24 h, an effect that seemed to be limited to nonsmokers and concluded that these data do not support a role for nicotine in perioperative analgesia. However, the results of this meta-analysis were limited by the heterogenicity of studies collected, as among the included nine studies TDN was used in six studies and nicotine nasal spray in three studies; hence, the effect of route of administration was neglected and TDN as a slow-release form provides different nicotine blood level and subsequent longer duration of action [Citation36].
These results were in agreement with the findings of Radwan et al. who reported that pre-emptive oral dose of 6 mg of melatonin reduced the pain scores and pethidine requirements in the first postoperative 24 h in patients undergoing abdominal surgery [Citation37].
Borazaa et al. also documented that preoperative oral melatonin 6 mg, the night before and 1 h before surgery, decreased pain scores and tramadol consumption and enhanced sleep quality and sedation scores during the postoperative period in patients undergoing elective prostatectomy [Citation38]. These results were consistent with Caumo et al. who found that 5 mg oral melatonin, the night before and 1 h before surgery in patients undergoing abdominal hysterectomy, decreased pain and anxiety during the first 24 h after surgery [Citation39].
These results were partially consistent with Khezri and Merate who reported that sublingual melatonin 3 mg premedication (60 min before surgery) for patients undergoing cataract surgery under topical anesthesia reduced the anxiety scores in patients and they could not demonstrate that melatonin has a decreasing effect on pain scores [Citation40].
The significant increase in the postoperative patient’s satisfaction score in TDM group as compared with the group C could be a consequence of melatonin’s effects on pain and anxiety which enhance sleep wake cycle disruption in stressful situations such as surgeries, providing better recovery quality [Citation38]. Clinical trials on the effect of melatonin on delirium in hip fracture patients are going on. Melatonin has been used successfully to treat and prevent postoperative delirium [Citation40]. Also, the significant increase in the postoperative patient’s satisfaction score in TDN group as compared with the group C was supported by the study by Ali and Sakr who documented that the higher response shown in their study in nicotine group versus control group was marginally significant [Citation34].
No significant differences in HR between the three groups were recorded at any time. There was a significant decrease in the mean arterial blood pressure in group (III) compared to groups (I) and (II) 30 min, 1 h, 2 h and 6 h postoperatively. Although nicotine has the potential to increase heart rate and blood pressure because it activates autonomic as well as central cholinergic receptors [Citation39], the current study did not show any significant difference in postoperative heart rate or blood pressure measurements with transdermal nicotine application versus placebo treatments. These results coincide with previous studies by Flood and Daniel; Habib et al.; Hong et al.; Turan et al. and Ali and Sakr in which the authors hypothesized that autonomic stimulation may have been offset by the fact that patients treated with nicotine were in less pain than those treated with placebo [Citation41,Citation28,Citation42,Citation43,Citation34].
In contrast to our results, the study by Ahmed Nagy and ElKadi who found that the sympathetic activating properties of nicotine with concomitant tachycardia and elevation of blood pressure were found to be advantageous for competing epidural sympatholytic effect and hence minimized decrease in blood pressure [Citation33].
These results were supported by the study by Ismail and Mowafi who reported that the mean arterial pressure was decreased after melatonin pre-medication and extended to the early postoperative period. This mild hypotensive effect of melatonin may be beneficial in elderly patients, particularly those at cardiovascular risk [Citation22].
Previous studies showed that melatonin could decrease MAP in healthy women [Citation44] and men [Citation45]. The mechanism of action of melatonin on circulation is complex and unclear. Melatonin may bind to specific melatonin receptors in the blood vessels, interfering with the vascular response to catecholamine [Citation46]. Furthermore, melatonin may interfere with the peripheral and central autonomic system, causing a reduction in adrenergic outflow and catecholamines levels [Citation47]. In addition, it may induce relaxation of the smooth muscle of the arterial walls via increasing nitric oxide availability [Citation48].
Intraoperative bleeding was significantly less in TDM group as compared to C and TDN groups. Intraoperative hypotension effectively decreases surgical blood loss and improves surgical field exposure which is essential for spinal surgeries. This result explained significant increase in the surgeon’s satisfaction score in TDM group compared to C and TDN groups.
As regards side effects in our study, all cases of the 3 groups were hemodynamically stable, no patient developed hypoxia and there were no reported intraoperative complications interfering with the course of surgery or interrupting the surgeons. Two patients in the C group suffered from nausea (p > 0.05). In the TDN group, the postoperative nausea and vomiting (PONV) occurred as follows: nausea (8 cases, p < 0.05) and vomiting (4 cases, p < 0.05), in spite of preoperative granisetron (1 mg IV) was given as a prophylactic antiemetic and there were no reported cases of erythema, respiratory depression or pruritus. In the TDM group, three patients were dizzy (p > 0.05).
These results were coincident with the findings of Greenland et al. who documented a meta-analysis involving patients participating in randomized trials of transdermal nicotine replacement therapy suggested that acute nicotine exposure can cause nausea in some settings and sleep disturbances [Citation49].
The frequency of occurrence of PONV was in agreement with the findings of Habib et al. (n = 29/44, v = 7/44 cases), Vibe Nielsen et al. and Ahmed Nagy and ElKadi (n and v = 18/50 cases) who documented that nicotine seems to increase the occurrence of nausea and vomiting [Citation28,Citation50,Citation33].
As regards adverse effects in patients who received TDM, patients were more sedated (P < 0.05) and two patients were dizzy (p > 0,05). These results were nearly similar to the results proved by Ismail and Mowafi who found that one patient in their melatonin group ((1/20 cases) complained of dizziness [Citation22].
Limitations of the study
Our study presented several limitations. First, the results did not allow for an evaluation of the effectiveness of TDN or TDM in all types of surgery. The second limitation was the small sample size preventing achievement safety conclusions. The third limitation of our study was that we did not measure nicotine and melatonin plasma levels so the interaction between both agents and endogenous melatonin could not be assessed. The fourth limitation was the use of one concentration of the nicotine patch; therefore, we were unable to establish whether there is a dose–response relationship for the analgesic effect of nicotine. The fifth limitation of our study was the patient population including nonsmoking women only. The results might not be, therefore, extrapolated to men or to smokers.
Conclusion
Transdermal administration of nicotine (15 mg/16 h) or transdermal therapeutic system containing 7 mg of melatonin 2 h preoperatively was an effective, simple, noninvasive, convenient technique and a safe adjuvant for acute postoperative pain after laparoscopic cholecystectomy. TDN and TDM allowed delivery of a potent analgesic agent providing a stable serum concentration for an extended period of time with acceptable minimal side effects. Prophylactic antiemetics were advocated to guard against the high possibility of development of nausea and/or vomiting after application of TDN patch.
Recommendations
The use of the TDN or TDM patches as a component of a multimodal analgesic system (i.e. in conjunction with regional blocks, a non-steroidal anti-inflammatory drug (NSAID), an acetaminophen or an α-agonist) may provide an effective postoperative analgesic regimen.
Clinical trial registration
ClinicalTrials.gov Identifier: NCT02747628.
Conflict of interest
None.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- P.D.WallThe prevention of postoperative painPain331988289290
- J.M.DesboroughThe stress response to trauma and surgeryBr J Anesth852000109117
- H.LauD.C.BrooksPredictive factors for unanticipated admissions after ambulatory laparoscopic cholecystectomyArch Surg136200111501153
- T.BisgaardJ.RosenbergH.KehletFrom acute to chronic pain after laparoscopic cholecystectomy: a prospective follow-up analysisScand J Gastroenterol40200513581364
- B.BernerV.A.JohnPharmacokinetic characterization of transdermal delivery systemsClin Pharmacokinet261994121134
- Y.ShiT.N.WeingartenC.B.MantillaW.M.HootenD.O.WarnerSmoking and pain: pathophysiology and clinical implicationsAnesthesiology1132010977992
- M.K.ChristensenD.F.SmithAntinociceptive effects of the stereoisomers of nicotine given intrathecally in spinal ratsJ Neural Transm Gen Sect801990189194
- T.J.RowleyJ.PayappillyJ.LuP.FloodThe antinociceptive response to nicotinic agonists in a mouse model of postoperative painAnesth Analg107200810521057
- F.BerrenderoV.MendizabalP.RobledoL.GaleoteA.Bilkei-GorzoA.ZimmerR.MaldonadoNicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin geneJ Neurosci25200511031112
- S.R.ChenH.L.PanSpinal endogenous acetylcholine contributes to analgesic effect of systemic morphine in ratsAnesthesiology952001525530
- A.HaghparastA.KhaniN.NaderiA.M.AlizadehF.MotamediRepeated administration of nicotine attenuates the development of morphine tolerance and dependence in micePharmacol Biochem Behav882008385392
- F.J.MiaoP.G.GreenN.BenowitzJ.D.LevineCentral terminals of nociceptors are targets for nicotine suppression of inflammationNeuroscience1232004777784
- H.WangM.YuM.OchaniC.A.AmellaM.TanovicS.SusarlaJ.H.LiH.WangH.YangL.UlloaY.Al-AbedC.J.CzuraK.J.TraceyNicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammationNature4212003384388
- L.C.OlsonD.HongJ.S.Conell-PriceS.ChengP.FloodA transdermal nicotine patch is not effective for postoperative pain management in smokers: a pilot dose-ranging studyAnesth Analg109200919871991
- L.F.SteadCochrane Database Syst Rev12008CD000146
- J.ArendtIn Melatonin and the mammalian pineal gland1995Chapman & HallLondon42
- J.Barrenetx eP.DelagrangeJ.A.MartinezJ Physiol Biochem60,612004
- F.WaldhauserM.WaldhauserH.R.LiebermanM.H.DengH.J.LynchR.WurtmanJ Neuroendocrinol391984307313
- B.LeeK.ParrottJ.AyresR.SackRes Commun Mol Path Pharmacol8531994337346
- B.KücükakinModification of surgical stress response by perioperative melatonin administrationDan Med Bull5752010B4144
- P.K.ZahnT.LansmannE.BergerE.SpeckmannU.MusshoffGene expression and functional characterization of melatonin receptors in the spinal cord of the rat: implications for pain modulationJ Pineal Res3520032431
- S.A.IsmailH.A.MowafiMelatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesiaAnesth Analg108200911461151
- M.L.LakinC.H.MillerM.L.StottW.D.WintersInvolvement of the pineal gland and melatonin in murine analgesiaLife Sci29198125432551
- N.-C.HjortsoN.J.ChristensenT.AndersenH.KehletEffects of the extradural administration of local anesthetic agents and morphine on the urinary excretion of cortisol, catecholamines, and nitrogen following abdominal surgeryBr J Anesth571985400406
- R.R.RikerJ.T.PicardG.L.FraserProspective evaluation of the sedation–agitation scale for adult critically ill patientsCrit Care Med27199913251329
- D.L.StreinerG.R.NormanScaling responsesD.L.StreinerG.R.NormanHealth measurement scales: a practical guide to their development and use1995Oxford University PressOxford2853
- T.RathjenU.BockmuhlC.A.GreimModern anesthesiologic concepts supporting paranasal sinus surgeryLaryngorhinootologie8520062023
- A.S.HabibW.D.WhiteM.A.El GasimG.SalehT.J.PolascikJ.W.MoulT.J.GanTransdermal nicotine for analgesia after radical retropubic prostatectomyAnesth Analg10720089991004
- A.M.HussainF.A.KhanA.AhmedT.ChawlaS.I.AzamEffect of gender on pain perception and analgesic consumption in laparoscopic cholecystectomy: an observational studyJ Anesthesiol Clin Pharmacol292013337341
- A.ZeidanS.Al-TemyattH.MowafiT.GhattasGender-related difference in postoperative pain after laparoscopic Roux-En-Y gastric bypass in morbidly obese patientsObes Surg23201318801884
- L.D.JamnerS.S.GirdlerD.ShapiroM.E.JarvikPain inhibition, nicotine, and genderExp Clin Psychopharmacol6199896106
- E.J.RichardsonT.J.NessD.T.ReddenC.C.StewartJ.S.RichardsEffects of nicotine on spinal cord injury pain vary among subtypes of pain and smoking status: results from a randomized, controlled experimentJ Pain13201212061214
- H.I.Ahmed NagyH.W.ElKadiTransdermal nicotine patch as adjunctive analgesic modality to thoracic epidural analgesia for post-thoracotomy painEgypt J Cardiothorac Anesth820147582
- A.R.AliS.A.SakrPre-emptive use of transdermal nicotine patch in lumber disc surgery: it’s effects on intraoperative anesthetic and analgesic requirements, hemodynamics and postoperativeAnalgesia Aust J Basic Appl Sci32200910961103
- B.YagoubianJ.AkkaraP.AfzaliD.M.AlfiL.OlsonJ.Conell-PriceNicotine nasal spray as an adjuvant analgesic for third molar surgeryJ Oral Maxillofac Surg69201113161319
- B.M.MishrikyA.S.HabibNicotine for postoperative analgesia: a systematic review and meta-analysisAnesth Analg1192014268275 [Epub ahead of print]
- Radwan K, Youssef M, ElTawdy A, Zeidan M, Kamal N. Melatonin versus Gabapentin. A comparative study of preemptive medications. The Internet Journal of Anesthesiology 2010;23 Number 1. http://doi:10.5580/265See more at: http://www.ispub.com/journal/ the internet journal of anesthesiology/volume23number1/ melatonin versus gabapentin a comparative study as preemptive medications. html#sthash.t GPya1v Y. dpuf.
- H.BorazaaS.TuncerN.YalcinA.ErolS.OtelciogluEffects of preoperative oral melatonin premedication on postoperative analgesia, sleep quality and sedation in patients undergoing elective prostatectomy: a randomized clinical trialJ Anesth242010155160
- W.CaumoF.TorresN.L.MoreiraJr.J.A.AuzaniC.A.MonteiroG.LonderoThe clinical impact of pre-operative melatonin on post-operative outcomes in patients undergoing abdominal hysterectomyAnesth Analg105200712631271
- M.B.KhezriH.MerateThe effects of melatonin on anxiety and pain scores of patients, intraocular pressure, and operating conditions during cataract surgery under topical anesthesiaIndian J Ophthalmol612013319324
- P.FloodD.DanielIntranasal nicotine for postoperative pain treatmentAnesthesiology101200414171421
- D.HongJ.C.PriceS.ChengP.FloodTransdermal nicotine patch for postoperative pain management: a pilot dose-ranging studyAnesth Analg107200810051010
- A.TuranP.F.WhiteO.KoyuncuB.KaramanliooluG.KayaC.C.ApfelTransdermal nicotine patch failed to improve postoperative pain managementAnesth Analg107200810111017
- A.De JongheB.C.Van MunsterH.E.van OostenJ.C.GoslingsP.KloenC.van ReesThe effects of melatonin versus placebo on delirium in hip fracture patients: study protocol of a randomized, placebocontrolled, double blind trialBMC Geriatr11201134
- A.CagnacciS.AranginoM.AngiolucciE.MaschioG.B.MelisInfluences of melatonin administration on the circulation of womenAm J Physiol2741998R335R338
- S.AranginoA.CagnacciM.AngiolucciA.M.VaccaG.LonguA.VolpeG.B.MelisEffects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy menAm J Cardiol83199914171419
- E.SewerynekMelatonin and the cardiovascular systemNeuro Endocrinol Lett23suppl 120027983
- R.M.BuijsS.E.La FleurJ.WortelC.Van HeyningenL.ZuiddamT.C.MettenleiterA.KalsbeekK.NagaiA.NiijimaThe suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neuronsJ Comp Neurol46420033648
- S.GreenlandM.H.SatterfieldS.F.LanesA meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patchDrug Saf181998287308
- S.Vibe NielsenR.H.PetersenA.PachaiA possible analgesic effect of nicotine on postoperative painUgeskr Laeger174201215941598