Abstract
Background
The field of cochlear implantation has been expanding rapidly and it has been hailed as one of the greatest advances in otology. The technique of anesthesia plays a crucial role in success of cochlear implant surgery as the anesthesiologist has to produce conditions which facilitate surgery by inducing bloodless operative field.
Study objective
To determine the efficacy of dexmedetomidine versus esmolol usage as an adjunct to induce controlled hypotension in children undergoing cochlear implant surgery.
Design
Clinical trial study.
Setting
Operating room in a university hospital.
Patients
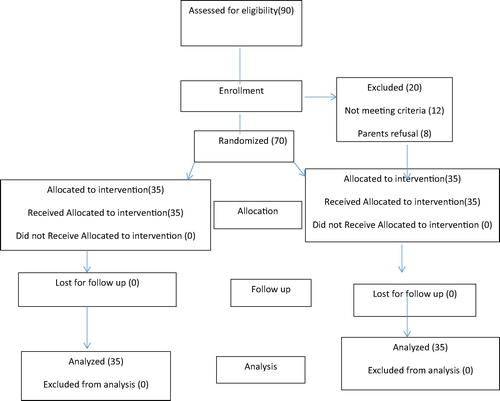
70 children aged 2–4 years scheduled for cochlear implant surgery under general anesthesia. Patients were randomly allocated according to drugs used into two equal groups (35 patients in each group). Interventions: Group (D): The patients in this group received a bolus dose of dexmedetomidine 0.5 ug/kg over 10 min followed by continuous infusion 0.2–0.5 ug/kg/h after induction of anesthesia but before surgery. Group (E): The patients in this group received a bolus dose of esmolol 0.5 mg/kg over 10 min followed by continuous infusion 100–300 ug/kg/min after induction of anesthesia but before surgery.
Measurements
Heart rate, Mean Arterial blood Pressure, Quality of surgical field, operative time, adverse events.
Main results
The quality of surgical field was comparable between both groups in all times of measurements. The time to first analgesic request was statistically significant longer in group (D) than in group (E) and the total tramadol consumption was statistically significant less in group (D) than in group (E).
Conclusions
In our study both dexmedetomidine and esmolol were effective in reducing MABP, and lowering the heart rate providing dry surgical field and ensured good surgical condition during cochlear implant surgery in pediatric patients.
Introduction
The field of cochlear implantation has been expanding rapidly and now it is an acceptable therapeutic option for those patients with irreversible hearing loss and deaf mutism. It has been hailed as one of the greatest advances in otology [Citation1].
The anesthesiologist is an integral member of the cochlear implant team whose anesthetic as well as communication skills are put to test. The technique of anesthesia plays a crucial role in success of cochlear implant surgery as the anesthesiologist has to produce conditions which facilitate surgery by inducing bloodless operative field, use of nerve stimulators and treat post-operative complications such as nausea, vomiting and vertigo [Citation2].
A bloodless surgical field is ideal for cochlear implant surgery, as even small amounts of blood will obscure the surgeon’s view in microsurgery. A combination of physical and pharmacologic techniques is used to minimize bleeding [Citation3].
Controlled hypotension is commonly used to achieve a bloodless operative field. Although the primary premise for its use is to limit intraoperative blood loss, an additional benefit is improved visualization of the surgical field [Citation4].
The use of controlled hypotension in pediatric surgery was first reported in 1953, thereafter, widely used in various pediatric surgical procedures, including scoliosis surgery, vascular surgery, and neurosurgery [Citation5].
In older children (9–18 years) undergoing functional endoscopic sinus surgery under controlled hypotension, no adverse outcomes were noted, and safe reduction in blood pressure (BP) has been regarded as a maximum of 25% below baseline mean BP [Citation6].
Various drugs have been used to induce controlled hypotension including vasodilators, alpha- and beta-adrenergic antagonist, beta-adrenergic antagonists, and high doses of potent inhaled anesthetics [Citation7].
While hypotension has proved safe to use in children, some develop tachycardia that delays the onset of hypotension. The introduction of the short acting beta-blocker esmolol enabled more precise control of heart rate.
Esmolol is an ultra-short acting intravenous cardio selective beta-antagonist. It has an extremely short elimination half-life and a total body clearance approaching 3 times of cardiac output and 14 times of hepatic blood flow [Citation8].
Dexmedetomidine is a specific and selective α2-adrenoceptor agonist. Drugs acting as agonists at α2-adrenoceptors may enhance anesthesia by providing dose-related sedation, anxiolysis, decreased upper airway secretions, perioperative hemodynamic stability and analgesia. There is substantial evidence that the α2-agonists also exert an anesthetic-sparing effect [Citation9].
The aim of this study was to determine the efficacy ofdexmedetomidine versus esmolol usage as an adjunct to induce controlled hypotension in children undergoing cochlear implant surgery. The primary outcomes are the quality of surgical field and surgical area bleeding score while duration of surgery and time to first analgesic request are the secondary outcomes.
Patients and methods
This randomized prospective double-blind study was conducted on 70 children aged 2–4 years scheduled for cochlear implant surgery under general anesthesia in the otorhinolaryngology department, Tanta University Hospital, after approval of the ethics committee and obtaining written informed consent from parents of each patient.
The approval code of ethics committee was 30290/05/15.
The randomization was performed using sealed numbered envelopes indicating the group of each patient. A blind nurse who did not participate in patients’ follow-up read the number and made group assignments. Study drugs were prepared by an independent anesthesiologist.
All patients’ data were confidential with secret codes and were used for the current study only.
Any unexpected risk appears during the course of the study was cleared to the guardian of the patient and the ethical committee on time and the proper measures were taken to minimize or overcome these risks.
.1 Inclusion criteria
Pediatric patients aging 2–4 years of either sex with ASA I and II scheduled for cochlear implant surgery.
.2 Exclusion criteria
Refusal to share in the study, known allergy to any of the study drugs, diabetes, liver and/or kidney disease, congenital heart disease and hemodynamic instability ().
.3 Preoperative preparation
All patients were underwent preoperative assessment by history taking, physical examination and laboratory investigations as needed.
.4 Intraoperative management
General anesthesia was induced by sevoflurane (7 vol.%,). After the patients’ loss of consciousness, intravenous line was inserted. Orotracheal intubation was facilitated by 1 μg/kg fentanyl and cisatracurium 0.15 mg/Kg and confirmed by clinical observation of chest wall movement, auscultation of chest and presence of square wave of capnogram. The patients were connected to mechanical ventilation. The respiratory rate and tidal volume were adjusted to maintain an ETCO2 between 32 and 35 mmHg.
Arterial catheter was inserted in the radial artery after Allen’s test for measurement of invasive blood pressure. Folly catheter was used to decompress the urinary bladder and to monitor urine output.
Anesthesia was maintained with sevoflurane 2–3 vol.% in 100 O2 and top up dose of cisatracurium 0.02 mg/kg every 30 min. Standard patient monitoring (electrocardiogram, non-invasive arterial pressure, heart rate, pulse oximetry, and end-tidal CO2) was used during anesthesia. All patients received a 5% dextrose in 0.45% saline at rate 5 ml/kg/h.
Facial nerve was identified intraoperatively by electrical stimulation after the effect of muscle relaxant has adequately reversed as evidenced by the nerve stimulator (train of four response); anesthesia was maintained during this stage by bolus dose of propofol 0.5 mg/kg and after the test was done muscle relaxant was given till the end of surgery.
After completion of surgery, inhalational anesthesia was stopped and muscle relaxant was reversed with atropine and neostigmine and the patient allowed to breathe spontaneously. The ETT was removed in deep plane of anesthesia to prevent coughing, bucking and sudden agitation which can displace the implant and the children were kept in recovery position and were transferred to postanesthesia care unit.
Metoclopramide 0.15 mg/kg and dexamethasone 0.15 mg/kg were administered for prophylaxis of postoperative nausea and vomiting (PONV) before the end of surgery. Ondansetron 0.1 mg/kg was administered for treatment of PONV.
.5 Randomization
The randomization was performed using sealed numbered envelopes indicating the group of each patient. A blind nurse who did not participate in patients’ follow-up read the number and made group assignments. Study drugs were prepared by an independent anesthesiologist.
The process of inclusion in the study went on until the required number of patients was reached. All operating room anesthesiologists, surgeons, and nurses were blinded to randomization, and preparations.
Patients were randomly allocated according to drugs used into two equal groups (35 patients in each group):
Group (D): The patients in this group received a bolus dose of dexmedetomidine (Precedex®, Meditera, 200 μg/2 mL) 0.5 ug/kg over 10 min followed by continuous infusion 0.2–0.5 ug/kg/h after induction of anesthesia but before surgery, in order to maintain the mean arterial blood pressure within 20–25% less than baseline reading.
Group (E): The patients in this group received a bolus dose of esmolol (Brevibloc®, Eczacibasi, 100 mg/10 mL) 0.5 mg/kg over 10 min followed by continuous infusion 100–300 ug/kg/min after induction of anesthesia but before surgery, in order to maintain the mean arterial blood pressure within 20–25% less than baseline reading. The infusions of study drugs were terminated 10 min prior to end of surgery to allow rise in blood pressure for effective hemostasis.
Measurements
| #x2022; | Demographic data: age, sex, ASA classification. | ||||
| #x2022; | Heart rate, Mean Arterial blood Pressure (preoperatively, after induction, then every 10 min). | ||||
| #x2022; | Quality of surgical field (by the operating surgeon every 30 min): with a predefined scale adapted from that of Dolman et al. [Citation10].
| ||||||||||
| #x2022; | Operative time. | ||||||||||
| #x2022; | Any adverse events. | ||||||||||
| #x2022; | Postoperative analgesia according to FLACC score [Citation11]. | ||||||||||
The pain intensity was assisted by a person who was blind to study by using FLACC scale [Citation11] graded from 0 to 10 (0 = no pain, 10 = the worst possible pain) in the following time 2 h, 4 h, 6 h, 8 h, 12 h, and 18 h after recovery.
able 1 FLACC scale.
Postoperative analgesia was given to all patients depending on pain score. If the value was less than 5, rectal paracetamol 20 mg/kg was given, if the value was more than 5, tramadol 1 mg/kg was given intravenously and recorded. The time to first dose of analgesia and total amount of tramadol used were recorded in all patients. Postoperative complications such as nausea, vomiting or bleeding were recorded.
Patients were discharged postoperatively when they had no or mild pain (FLACC < 3), were able to tolerate clear fluids and soft food and had no bleeding or nausea or vomiting.
.6 Statistical analysis
The sample size was calculated using the following assumption: the surgical area bleeding score was the main response variable, and medcalc program version 3.5 was used for sample size calculation.
Power analysis identified 32 patients per group, required to detect 15% difference between groups with a power 80% and a significant level of 0.05. However, to enable detection of potential variations between the two groups and avoid potential errors, 35 patients were included in each group.
Comparison of demographic data and time of surgery was done by Student’s t-test. Two way analysis of variance with correction for repeated measurements was used for heart rate and blood pressure comparison. Mann–Whitney U test was used for nonparametric measurements including quality of surgical field and pain score. P < 0.05 was considered significant.
Results
This study was carried out on 70 patients divided into two groups, 35 in each group. The groups were comparable with regard to demographic data including age, weight, and duration of surgery. The Time to first analgesic request was statistically significant longer in group (D) than in group (E) and the total tramadol consumption was statistically significant less in group (D) than in group (E) [].
able 2 Demographic data; duration of surgery, tramadol consumption; time of first analgesic requirement.
Pain score after 2 h was statistically insignificant between both groups (P > 0.05), while pain score at 4 h, 6 h, and 8 h in group D was significantly less when compared to group E (P < 0.05) .
able 3 The value of pain score.
Pain score at 12 h and 18 h was comparable between both groups p > 0.05 ().
The quality of surgical field was comparable between both groups in all times of measurements p > 0.05 .
able 4 Quality of surgical field.
The MABP and HR were comparable between both groups in all times of measurements, p value > 0.05 and the values in both groups were decreased significantly after infusion of the study drugs till the end of surgery when compared to baseline and .
able 5 Changes in MABP.
able 6 Changes in HR.
Discussion
Our study demonstrated that, both dexmedetomidine and esmolol were effective in reducing blood pressure, heart rate, minimizing surgical site bleeding, improving quality of surgical field, better visualization of the surgical field, and decreasing operative time with no reported complications in children underwent cochlear implant surgery.
Intraoperative goals are to maintain stable hemodynamics, to provide immobile bloodless field, modulation of anesthetic technique to allow facial nerve monitoring and to reduce interference with stapedius reflex testing. Measures should be taken to prevent postoperative nausea and vomiting and adequate analgesia should be provided [Citation12].
Dexmedetomidine is a highly specific and selective alpha-2-adrenergic agonist with sedative, anxiolytic, and organ protective effects. Its clinical applications in children include premedication, prevention of emergence delirium, as part of multimodal anesthetic regimen and sedation in the pediatric intensive care unit [Citation13–Citation15].
Dexmedetomidine has anesthetic, analgesic, and sympatholytic properties [Citation16–Citation18]. The sympatholytic effect is manifested by decreases in arterial blood pressure (BP), heart rate (HR), and norepinephrine release. Thus, dexmedetomidine has the potential to attenuate perioperative increases in BP and HR [Citation19,Citation20].
The probable mechanism of reducing blood pressure by dexmedetomidine is attributed to stimulation of peripheral alpha2 adrenoceptors of vascular smooth muscle. This results in decrease in blood pressure and heart rate secondary to inhibition of central sympathetic out flow.
A randomized study investigating the effectiveness of dexmedetomidine in reducing bleeding during septoplasty and tympanoplasty operations demonstrated dexmedetomidine significantly reduces bleeding and fentanyl requirement in septoplasty tympanoplasty operations [Citation15].
Durmus and colleagues [Citation21] used dexmedetomidine to improve the quality of surgical field in both tympanoplasty and septoplasty, and concluded that dexmedetomidine is a useful adjuvant to decrease bleeding.
Esmolol is an ultra-short acting intravenous cardioselective beta-antagonist. It has an extremely short elimination half-life (mean: 9 min; range 4–16 min) and a total body clearance approaching 3 times of cardiac output and 14 times of hepatic blood flow [Citation8].
The hypotensive effect of esmolol was a balanced result between their direct cardiac effect and/or vasomotor effect and the vasomotor drives originating from the counter-regulatory responses. Also the hypotensive effect of esmolol is due to decrease in cardiac output, and the reduction in heart rate [Citation8].
Esmolol can be administered safely to patients younger than 6 years after repair of coarctation of the aorta. In the dose range of 125–500 microg/kg, esmolol significantly decreased systolic blood pressure [Citation22].
Studies [Citation23,Citation24] have shown that induced hypotension with a beta blocking agent enhanced norepinephrine, endocrine and metabolic responses of small magnitude during middle ear surgery; this attested that there was an increase of the sympathetic tone [Citation25,Citation26] leading to vasoconstriction of arterioles andprecapillary sphincters that resulted unopposed alpha-adrenergic effects during esmolol hypotension. However, based on their known pharmacological effects, beta blockers decrease cardiac output and therefore decrease the flow to the tissue. However, beta blockade would only be appropriate for capillary bleeding [Citation27].
The time to first analgesic requirement was shorter in esmolol group and amount of analgesic requirements was less in dexmedetomidine group.
El Saied MH found that, Dexmedetomidine infusion in cochlear implantation in pediatric patients was better in inducing deliberate hypotension and providing better quality scale of surgical field compared to fentanyl infusion. It allowed rapid recovery from anesthesia and reduced need for pain medication in the PACU [Citation28].
Ibraheim OA et al., found that both esmolol and dexmedetomidine, added to anesthetic regimen, provided an effective and well-tolerated method to reduce the amount of blood loss in patients undergoing scoliosis surgery [Citation29].
Dikmen et al., found that infusion of dexmedetomidine was effective in inducing consistent and sustained controlled hypotension, and achieved clear surgical field during middle ear surgery with no need for additional use of a potent hypotensive agent in low-flow anesthesia. Dexmedetomidine also reduced isoflurane and fentanyl requirements for deliberate hypotension and attenuated cardiovascular responses perioperatively [Citation30].
Tobias and Berkenbosch found that, Dexmedetomidine was an effective agent for controlled hypotension during anterior spinal fusion without the need to use a beta adrenergic antagonist to control heart rate as is sometimes the case with direct acting vasodilators [Citation31].
Nasreen et al., concluded that Dexmedetomidine can be safely administered to provide hypotensive anesthesia during middle ear surgery [Citation32].
In line with our findings, Feld et al. [Citation33] reported that dexmedetomidine provided stable postoperative analgesia, thus reducing the use of morphine in the postoperative period when comparing fentanyl and dexmedetomidine combined with desflurane for bariatric surgery.
Ibraheim et al., found that fentanyl and propofol consumption were significantly lower in the dexmedetomidine group compared with the esmolol and control groups [Citation29].
Unlugenc H, who found that a single i.v. dose of dexmedetomidine (1 microg/kg) given 10 min before induction of anesthesia significantly reduced postoperative morphine consumption and had no effect on postoperative recovery time [Citation34].
Dexmedetomidine significantly reduces the requirements for rescue sedation by 80% and analgesia by 50% in postoperative patients for up to 24 h. Its sedative properties differ from traditional agents with patients being more easily roused [Citation35].
Gurbet et al., found that Continuous iv dexmedetomidine during abdominal surgery provides effective postoperative analgesia, and reduces postoperative morphine requirements without increasing the incidence of side effects [Citation36].
Our study found no significant difference between both groups as regard postoperative nausea and vomiting.
However, Ali et al. [Citation37] reported the incidence of postoperative nausea and vomiting was less in pediatric patients receiving dexmedetomidine in comparison with those receiving fentanyl during extracorporeal shock wave lithotripsy. Also, the same results were reported by Turgut et al. [Citation38] in adult patients undergoing lumbar laminectomy.
The limitations of the present study, include the following: no control group, we did not measure the amount of blood loss, the scale used for assessing the quality of surgical site bleeding was subjective, and we did not measure the depth of anesthesia. Further study was needed to compare the dexmedetomidine with other agent used for controlled hypotensive anesthesia in children.
Conclusion
Our study demonstrated that both dexmedetomidine and esmolol are safe agents for inducing controlled hypotension and both are effective in providing ideal surgical field in pediatric patients undergoing cochlear implant surgery with no reported complications. Dexmedetomidine offers the advantage over esmolol and it prolongs postoperative analgesia and decreases opioid used postoperatively.
Conflict of interest disclosure
Have no conflict and No fund.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- C.B.PedersenU.JochumsenS.MadsenB.Koefoed-NielsenL.V.JohansenResults and experiences with 55 cochlear implantationsUgeskr Laeger16240200053465350
- L.C.A.ChakrabartyS.C.TarnejaS.V.A.SinghB.RoyC.BhargavaL.C.SreevastavaCochlear implant: anaesthesia challengesMed J Armed Forces India6042004351356
- G.B.AydinO.OzluH.AlacakirM.AksoyControlled hypotension: remifentanil or esmolol during tympanoplastyMediterr J Otol422008125131
- C.S.DegouteM.J.RayM.ManchonC.DubreuilV.BanssillonRemifentanil and controlled hypotension; comparison with nitroprusside or esmolol during tympanoplastyCan J Anaesth48120012027
- T.C.K.BrownEarly experiences of vasodilators and hypotensive anesthesia in childrenPediat Anesthesia222012720722
- S.M.RagabM.Z.HassaninOptimizing the surgical …field in pediatric functional endoscopic sinus surgery: A new evidence-based approachOtolaryngol Head Neck Surg142120104854
- C.S.DegouteControlled hypotension: a guide to drug choiceDrugs67200710531076
- Wiest.D.EsmololA review of its therapeutic efficacy and pharmacokinetic characteristicsClin Pharmacokinet281995190202
- M.G.CoughlanJ.G.LeeZ.J.BosnjakW.T.SchemelingJ.P.KampineD.C.WarltierDirect coronary and cerebral vascular responses to dexmedetomidine. Significance of endogenous nitric oxide synthesisAnesthesiology7719929981006
- R.M.DolmanK.C.BentleyT.W.HeadM.EnglishThe effect of hypotensive anesthesia on blood loss and operative time during Le Forte osteotomiesJ Oral Maxillofac Surg582000834839
- MerkelVoepel-LewisThe FLACC: a behavioral scale for scoring postoperative pain in young childrenPediat Nurs231997293
- BaidyaDalim KumarDehranMayaAnaesthesia for cochlear implant surgeryTrends Anaesth Crit Care1220119094
- T.J.EberJ.E.HallJ.A.Bar neyThe effects of increasing plasma concentrations of dexmedetomidine in humansAnesthesiology9322000382394
- P.TalkeC.A.RichardsonM.ScheininD.M.FisherPostoperative pharmacokinetics and sympatholytic effects of dexmedetomidineAnesth Analg855199711361142
- H.AyogluO.YapakciM.B.UgurL.UzunH.AltunkayaY.OzerEffectiveness of dexmedetomidine in reducing bleeding during septoplasty and tympanoplasty operationsJ Clin Anesth2062008437441
- B.C.BloorD.S.WardJ.P.BellevilleM.MazeEffects of intravenous dexmedetomidine in humans. II. Hemodynamic changesAnesthesiology77199211341142
- J.P.BellevilleD.S.WardB.C.BloorM.MazeEffects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rateAnesthesiology77199211251133
- M.AhoA.M.LehtinenO.ErkolaThe effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomyAnesthesiology7419919971002
- J.W.FlackeB.C.BloorW.E.FlackeD.WongS.DazzaS.W.SteadReduced narcotic requirement by clonidine with improved hemodynamic and adrenergic stability in patients undergoing coronary bypass surgeryAnesthesiology6719871119
- M.F.RoizenShould we all have a sympathectomy at birth? Or at least preoperatively?Anesthesiology681988482484
- M.DurmusA.K.ButZ.DoganA.YucelM.C.MimanM.O.ErsoyEffect of dexmedetomidine on bleeding during tympanoplasty or septorhinoplastyEur J Anaesthesiol2452007447453
- S.TabbuttS.C.NicolsonP.C.AdamsonX.ZhangM.L.HoffmanW.WellsThe safety, efficacy, and pharmacokinetics of esmolol for blood pressure control immediately after repair of coarctation of the aorta in infants and children: a multicenter, double-blind, randomized trialJ Thorac Cardiovasc Surg13622008321328
- G.V.DietrichM.HeesenJ.BoldtG.HempelmannPlatelet function and adrenoceptors during and after induced hypotension using nitroprussideAnesthesiology85199613341340
- W.S.BlauE.R.KaferJ.A.AndersonEsmolol is more effective than sodium nitroprusside in reducing blood loss during orthognathic surgeryAnesth Analg751992172178
- C.S.DegouteC.DubreuilM.J.RayJ.GuittonM.ManchonV.BanssillonEffects of posture, hypotension and locally applied vasoconstriction on the middle ear microcirculation in anaesthetized humansEur J Appl Physiol691994414420
- A.P.BoezaartJ.Van der MerweA.CoetzeeComparison of sodium nitroprusside and esmolol-induced controlled hypotension for functional endoscopic sinus surgeryCan J Anaesth421995373376
- Y.J.LimC.S.KimJ.H.BahkB.M.HamS.H.DoClinical trial of esmolol induced controlled hypotension with or without acute normovolemic hemodilution in spinal surgeryActa Anaesthesiol Scand4720037478
- M.H.El SaiedN.N.MohamedH.M.MohamedM.I.AminDexmedetomidine versus fentanyl in anesthesia of cochlear implantation in pediatric patientsEgypt J Anaesth3220165559
- O.A.IbraheimA.AbdulmonemJ.BaajT.A.ZahraniV.ArletEsmolol versus dexmedetomidine in scoliosis surgery: study on intraoperative blood loss and hemodynamic changesMiddle East J Anaesthesiol22120132733
- B.DikmenF.SahinD.OrnekY.PalaO.KilciE.HorasanliM.CanturkDexmedetomidine for controlled hypotension in middle ear surgery with low-flow anesthesia controlled hypotension with low-flow anesthesiaInt Adv Otol632010331336
- D.J.TobiasW.J.BerkenboschInitial experience with dexmedetomidine in paediatric-aged patientsPediat Anaesth122002171175
- F.NasreenS.BanoR.M.KhanS.A.HasanDexmedetomidine used to provide hypotensive anesthesia during middle ear surgeryInd J Otolaryngol Head Neck Surg6132009205207
- J.M.FeldW.E.HoffmanM.M.StechertI.W.HoffmanR.C.AnandaFentanyl or dexmedetomidine combined with desflurane for bariatric surgeryJ Clin Anesth18120062428
- H.UnlugencM.GunduzT.GulerO.YagmurG.IsikThe effect of pre-anaesthetic administration of intravenous dexmedetomidine on postoperative pain in patients receiving patient-controlled morphineEur J Anaesthesiol2252005386391
- R.M.VennC.J.BradshawR.SpencerD.BrealeyE.CaudwellC.NaughtonPreliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unitAnaesthesia5412199911361142
- A.GurbetE.Basagan-MogolG.TurkerF.UgunF.N.KayaB.OzcanIntraoperative infusion of dexmedetomidine reduces perioperative analgesic requirementsCan J Anaesth5372006646652
- A.AliM.El GhoneimyDexmedetomidine versus fentanyl as adjuvant to propofol: comparative study in children undergoing extracorporeal shock wave lithotripsyEuro J Anesthesiol27201010581064
- N.TurgutA.TurkmenS.GökkayaA.AltanM.A.HatibogluDexmedetomidinebased versus fentanyl-based total intravenous anesthesia for lumber laminectomyMinerva Anesthesiol742008469474