Abstract
Background
Delirium is a common problem among intensive care patients and can contribute to non-invasive ventilation (NIV) failure. Prevention of delirium may improve the outcome and success rate of NIV. The aim of the current study was to compare the effects of using dexmedetomidine and haloperidol for prevention of delirium during NIV.
Patients and methods
Ninety adult intensive care patients of ASA physical status III and IV on NIV were randomly allocated to three equal groups. Group D (30 patients) received dexmedetomidine continuous intravenous infusion of 0.2–0.7 μg/kg/h preceded by a loading dose of 1.0 μg/kg intravenously over 10 min if needed, group H (30 patients) received haloperidol continuous intravenous infusion of 0.5–2 mg/h preceded by a loading dose of 2.5 mg intravenously if needed and group P (30 patients) received normal saline infusion. Supplementary sedation or analgesia was given when needed. Our primary outcome measure was the incidence of delirium. Duration of non-invasive mechanical ventilation, incidence of endotracheal intubation during NIV, length of ICU stay, and length of hospital stay, adverse events and mortality were recorded. Delirium was diagnosed using the Confusion Assessment Method for the ICU (CAM-ICU). Hemodynamic parameters and respiratory rate were recorded.
Results
The incidence of delirium was significantly lower in dexmedetomidine group 3/30 (10%) than haloperidol 10/30 (33.3%) and placebo groups 13/30 (43.3%) groups. Duration of NIV was significantly shorter in dexmedetomidine group than in placebo group and shorter than haloperidol group. The incidence of endotracheal intubation was significantly less in dexmedetomidine group compared to placebo and haloperidol groups. The length of ICU and hospital stay was significantly shorter in dexmedetomidine group compared to haloperidol and placebo groups Bradycardia occurred significantly more in dexmedetomidine group while prolonged corrected QT (QTc) interval occurred only in haloperidol group (2 patients). A significantly lower incidence of respiratory tract infections was noted in dexmedetomidine group. The need for supplementary sedatives and analgesic was significantly less in dexmedetomidine group compared to haloperidol and placebo groups.
Conclusion
Dexmedetomidine is more effective than haloperidol for prevention of delirium during NIV with lower incidence of endotracheal intubation and shorter ICU and hospital stay. Bradycardia is more frequent with dexmedetomidine use.
1 Introduction
Non-invasive ventilation (NIV) has an increasing role in intensive care due to its benefits in reducing dependence on invasive mechanical ventilation and associated complications. However, noninvasive ventilation failure remains a challenging aspect with rates approaching 40% in some studies [Citation1,Citation2]. Agitation, intolerance, and patient–ventilator asynchrony are factors implicated in NIV failure for which judicious sedation is recommended [Citation3].
Delirium is defined in the American Psychiatric Association’s (APA) as a disturbance of consciousness and cognition that develops over a short period of time (hours to days) and fluctuates over time [Citation4]. Delirium is classified to either hypoactive (not agitated), hyperactive (agitated), or mixed [Citation5]. Delirium is common in intensive care unit (ICU) especially among mechanically ventilated patients. A high prevalence of delirium and agitation in NIV patients (about 37%) is linked to a marked increase in the risk of NIV failure [Citation6]. Therefore, prevention of delirium in ICU patients may be beneficial in improving the outcome in these patients.
Prevention of delirium depends mainly on non-pharmacological methods, particularly early mobilization and noise reduction [Citation7]. The role of sedation in promotion or prevention of ICU delirium has attracted much comment in the recent years. Sedatives or opioids may improve patient comfort and tolerance during NIV but they may induce respiratory depression [Citation8] and sometimes difficult to titrate their pharmacologic effects because they may accumulate with repeated dosing. Benzodiazepines may be associated with a greater risk of delirium and cognitive impairment [Citation9,Citation10].
Dexmedetomidine is an alpha 2 adrenoreceptor agonist providing sedation and analgesia with attenuation of the stress response with no significant respiratory depression (12) [Citation11]. Dexmedetomidine was used for sedation and management of NIV failure in acute respiratory failure patients [Citation12]. Dexmedetomidine has several advantages over other medications such as narcotics, benzodiazepines, or propofol as a sedative in the ICU. Dexmedetomidine produces minimal respiratory depression, which facilitates sedation in the nonintubated patients [Citation13].
Haloperidol is a D2 receptors antagonist. Blockade of D2 receptors may result in improvement of hallucination and delusions. The use of haloperidol can also reduce the need for sedative and analgesic drugs in ventilated patients. It is recommended as the drug of choice to treat ICU delirium by the Society of Critical Care Medicine and American Psychiatry Association [Citation14]. Haloperidol has a number of side effects, including extrapyramidal manifestations and rarely neuroleptic malignant syndrome but the most dangerous adverse effect is prolongation of the corrected QT (QTc) interval which can precipitate fatal arrhythmias [Citation15,Citation16].
This study aimed to assess the effects of early use of dexmedetomidine and haloperidol on incidence of delirium in patients with NIV and its effects on the duration of NIV and incidence of endotracheal intubation.
2 Patients and methods
2.1 Study design
This prospective, randomized, double blinded controlled study was carried out after approval of our institutional review board (IRB) and obtaining informed consent from all patients or relatives. Ninety adult intensive care patients of ASA physical status III and IV aged between 26 and 70 years, in zagazig university hospital from January 2014 to October 2015 were included in this study. All patients were candidates for noninvasive mechanical ventilation (NIV). Patients were randomly allocated according to a computer generated random number into one of three groups (). Group D (30 patients) received dexmedetomidine infusion, group H (30 patients) received haloperidol infusion and Group P (30 patients) received normal saline infusion. All medications for the three groups were prepared and labeled by the pharmacist in 50 ml syringe pumps and they changed the concentration of the drugs during preparation according to the patient weight to keep the rate of infusion fixed for the three groups (10 ml for bolus dose and 2–8 ml/h for continuous infusion). All medications were given by the intensive care staff (physicians and nurses) that are not involved in the study and are unaware of the infused drugs for the three groups. Patients in dexmedetomidine (D) group received Dexmedetomidine continuous intravenous infusion of 0.2–0.7 μg/kg/h preceded by a loading dose of 1.0 μg/kg intravenously over 10 min if needed [if Richmond Agitation Sedation Scale (RASS) was +2 or more]. In haloperidol (H) group haloperidol was administered as a continuous intravenous infusion of 0.5–2 mg/h preceded by a loading dose of 2.5 mg intravenously over 10 min if needed [if Richmond Agitation Sedation Scale (RASS) was +2 or more]. Patients in group P received normal saline continuous intravenous infusion (2–8 ml/h) and a loading dose (10 ml) over 10 min if needed [if Richmond Agitation Sedation Scale (RASS) was +2 or more].
Supplementary doses of sedatives (midazolam or propofol) were given to any patient in the studied groups if RASS was +1 or more. Midazolam was given when RASS was +1 or +2 and propofol was given when RASS was +3 or +4 or when patients were still agitated after receiving midazolam. Analgesia (fentanyl) was given to any of the studied patients with VAS score ⩾5. The need for supplementary sedation or analgesia was recorded (the number of patients and the doses received).
Inclusion criteria were, age more than 18 years, patients need NIV due to acute exacerbation of acute respiratory failure in chronic obstructive pulmonary disease (COPD), patients with acute hypoxemic cardiogenic pulmonary edema and postoperative respiratory failure patients.
Exclusion criteria included patients or relatives refusal, patients with known allergy to any of the studied drugs, patients with known psychiatric disorders or on antipsychotic medications, patients with severe dementia, patients with heart rate ⩽50 beats/min or systolic blood pressure ⩽90 mmhg, patients with prolonged QTc-time (>500 ms) or history of clinically relevant ventricular arrhythmia, patients with epilepsy or parkinsonism and pregnancy.
Patients were connected to a ventilator (dragger evita4) (Germany) with a suitable size full face mask (Respironics, Monroeville, PA, USA). Initial settings of IPAP 10 cms H2O titrated in increments of 2–3 cm H2O (to a maximum of 20–25 cm H2O) at 5–10 min intervals over the first 30–60 min according to the clinical response and patient tolerance to obtain an exhaled tidal volume of 6–8 mL/kg and a respiratory rate (RR) less than 35 breaths min−1. EPAP was adjusted at 4–5 cms H2O and was gradually increased by 2 cm H2O to a maximum of 10 cm H2O until the FiO2 requirement was 65% or less to maintain the oxygen saturation above 92%. The ventilator settings were then adjusted on the basis of pulse oximetry and serial measurements of arterial blood gases.
Noninvasive ventilation (NIV) and drug sedation were reduced progressively according to the degree of clinical improvement and were discontinued if the patient stably maintained a RR < 25 breaths min−1 and a PaO2/FiO2 > 200.
Endotracheal intubation was indicated at any time during the study if patient refused or not tolerated noninvasive ventilation, if partial pressure of arterial carbon dioxide increased with a pH of ⩽7.20, if patient failed to maintain a PaO2/FiO2 ratio by greater than 150, with severe hemodynamic instability (defined as a systolic blood pressure of less than 70 mmHg, or evidence of ischemia or clinically significant ventricular arrhythmias) and for airway protection (e.g., seizure disorder or vomiting).
Our primary outcome measure was the incidence of delirium in patients with non-invasive mechanical ventilation. Secondary outcomes included, the duration of NIV, incidence of endotracheal intubation during NIV, length of ICU stay, length of hospital stay, adverse events and mortality.
All patients were monitored by electrocardiography, pulse oximetry, non-invasive blood pressure (SBP and DBP) and arterial blood gases. All complications were noted and recorded. Bradycardia was diagnosed if HR decreased to ⩽60 beats/min and it needed intervention if HR decreased to ⩽50 beats/min. Hypotension was diagnosed if SBP ⩽ 90 mmhg or DBP ⩽ 60 mmhg. Twelve lead electrocardiography done if prolonged QTc-time was suspected.
Delirium was diagnosed using the Confusion Assessment Method for the ICU (CAM-ICU) [Citation17]) where delirium is diagnosed in two steps (). First step was to assess the level of consciousness using the Richmond Agitation Sedation Scale (RASS) [Citation18] [which is a 10-point scale ranging from +4 to −5]. Patients with moderate sedation (RASS score −3) or more alert should be evaluated for delirium. The CAM-ICU assesses patients for four features of delirium; three out of four features are required for a diagnosis of delirium (). Confusion Assessment Method for the ICU (CAM-ICU) was performed every four hours.
2.2 Sample size calculation
Power analysis was performed using Chi square test for independent samples on frequency of patients complaining of delirium because it was the main outcome variable in the present study. A pilot study was done before starting this study because there were no available data in the literature for the incidence of delirium with dexmedetomidine administration during NIV. The results of the pilot study showed incidence of delirium of 8% in dexmedetomidine group, and 38% in control group. Taking power 0.8 and alpha error 0.05, a minimum sample size of 27 patients was calculated for each group. A total of patients in each group (30) were included to compensate for possible dropouts (MedCalc 13 for windows, MedCalc Software bvba, Ostend, Belgium).
3 Statistical analysis
Continuous variables were expressed as the mean ± SD and the categorical variables were expressed as a number (percentage). Continuous variables were checked for normality by using Shapiro-Wilk test. One way ANOVA test was used to compare three groups of normally distributed data, while Kruskall Wallis H was used for non-normally distributed data. Wilcoxon signed rank test was used to compare two dependent groups of non-normally distributed data. Percent of categorical variables was compared using the Chi-square test. All tests were two sided. P < 0.05 was considered statistically significant. All data were analyzed using Statistical Package for Social Science for windows version 20.0 (SPSS Inc., Chicago, IL, USA) & MedCalc for windows version 13 (MedCalc Software bvba, Ostend, Belgium) and graphically presented using Microsoft Office Excel 2010 for windows (Microsoft Cor., Redmond, WA, USA).
4 Results
Ninety adult intensive care patients were included in our study and randomized into three groups. There were no significant differences among the three groups regarding the baseline characteristics (p > 0.05) ().
Table 1 Baseline characteristics.
Regarding the primary and secondary outcomes () the incidence of delirium was significantly lower in dexmedetomidine group 3/30(10%) than haloperidol 10/30(33.3%) and placebo groups 13/30(43.3%) groups. Duration of non-invasive mechanical ventilation was significantly shorter in dexmedetomidine group (34.9 ± 8.2 h) than placebo group (52.9 ± 12.7 h) and shorter than haloperidol group (39.3 ± 10.2 h) with significantly more patients in placebo group (14 patients) and halo group (11 patients) required endotracheal intubation compared to dexmedetomidine group (4 patients).
Table 2 Primary and secondary outcomes in the three groups.
The length of ICU stay was significantly shorter in dexmedetomidine group (3.1 ± 0.4 days) compared to 6.5 ± 1.0 and 6.9 ± 1.2 days in halo and placebo groups respectively. Also the length of hospital stay was significantly shorter in dexmedetomidine group (6.2 ± 0.9 days) compared to 13.5 ± 2.0 and 15.5 ± 2.5 days in haloperidol and placebo groups respectively. The mortality was lower in both dexmedetomidine and haloperidol groups (2 patients in each group) while it was 3 patients in placebo group.
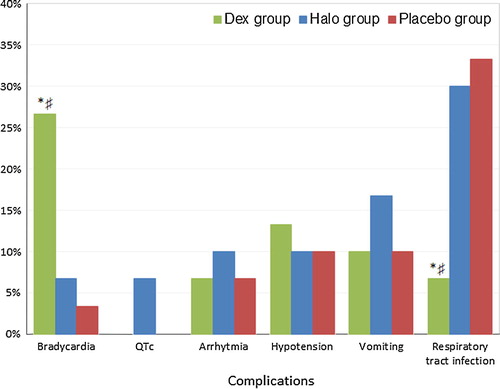
shows the incidence of complications occurred in the three studied groups. Bradycardia occurred significantly more in dexmedetomidine group (8 patients) than in haloperidol group (2 patients) and placebo group (1 patient). Two patients in haloperidol group developed prolonged QTc-interval (>500 ms) (confirmed with 12 lead e c g) which improved with holding haloperidol for 2 h. No patients in both placebo and dexmedetomidine groups developed Prolonged QTc interval. Three patients developed arrhythmia in haloperidol group compared to 2 patients in both dexmedetomidine and placebo groups. Hypotension occurred in 4 patients in dexmedetomidine group with dose reduction by 50% from the current dose in 3 of them while hypotension occurred in 3 patients in both haloperidol and placebo groups. More patients developed vomiting in haloperidol group (5 patients) compared to 3 patients in both dexmedetomidine and placebo groups. A significantly lower incidence of respiratory tract infections was noted in dexmedetomidine group (2 patients) compared to haloperidol (9 patients) and placebo groups (10 patients).
The need for supplementary sedatives and analgesics is shown in . A significantly lower number of patients (2 patients) in dexmedetomidine group needed midazolam with a total dose of 5.5 ± 1.0 mg than haloperidol group (8 patients) needed 13.5 ± 1.9 mg and placebo group (11 patients) needed 30 ± 4.8 mg. Also propofol was significantly less needed in dexmedetomidine group (3 patients) needed 320.2 ± 88.2 mg compared to haloperidol group (10 patients) needed 680.1 ± 162.2 mg and placebo groups (13 patients) needed 1151.4 ± 241.9 mg. Fentanyl was significantly less needed in dexmedetomidine group (2 patients needed 100.5 ± 29.4 mcg) compared to 8 patients (351.6 ± 88.2 mcg) in haloperidol group and 10 patients (480.1 ± 117.2 mcg) in placebo group.
Table 3 Supplementary sedatives and analgesic needs during NIV.
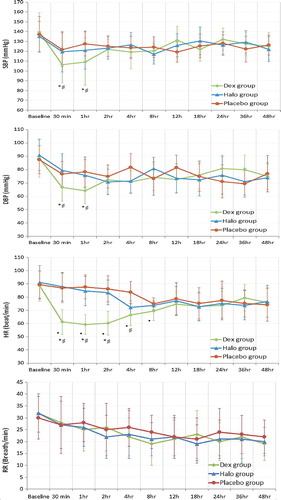
shows the hemodynamic parameters and respiratory rate in the 3 studied groups. There were no significant differences in baseline measurements between the three studied groups. Both SBP and DBP were significantly lower after 30 min and 1 h in dexmedetomidine group when compared to the baseline readings. Also SBP and DBP were significantly lower in dexmedetomidine group after 30 min and 1 h when compared to haloperidol and placebo groups. There was no significant difference between the 3 studied groups regarding the respiratory rate. Patients in dexmedetomidine group had a significantly lower H R for 8 h when compared to baseline measurement. Heart rate was significantly lower in dexmedetomidine group compared to the placebo group after 30 min, 1 h, 2 h, and 4 h. Also HR was significantly lower in dexmedetomidine group after 30 min, 1 h and 2 h compared to haloperidol group.
5 Discussion
Non-invasive mechanical ventilation supports breathing without the need for intubation and has the added advantage of lower needs for sedation. Prevention of agitation and delirium during NIV can decrease the incidence of endotracheal intubation and invasive mechanical ventilation.
In the present study we compared the effects of early prophylactic use of dexmedetomidine or haloperidol on the incidence of delirium during NIV. We also studied the effects of the studied drugs on the duration of NIV and the incidence of NIV failure with need of endotracheal intubation and the length of ICU and hospital stay.
The incidence of delirium was significantly lower in dexmedetomidine group than haloperidol and placebo groups. This may be due to the state of cooperative sedation and analgesia without effect on the respiratory drive produced by dexmedetomidine. These criteria with dexmedetomidine use contribute to less need for supplementary sedatives and analgesic that may have a role in occurrence of delirium. Incidence of delirium was also lower in haloperidol group compared to placebo group. In a study compared haloperidol with placebo as a prophylactic therapy for the prevention of postoperative delirium in elderly hip surgery patients the severity and duration of delirium were reduced but the incidence of delirium was not altered by haloperidol compared to placebo [Citation19].
Duration of non-invasive mechanical ventilation was significantly shorter in dexmedetomidine group than placebo group with more patients required endotracheal intubation in placebo group (14 patients) compared to dexmedetomidine group (4 patients). Also the duration of NIV and number of patients required endotracheal intubation was less in dexmedetomidine group than haloperidol group. The length of ICU stay and the hospital stay were significantly shorter in dexmedetomidine group compared to haloperidol and placebo groups. This reduced duration of NIV and incidence of endotracheal intubation with shorter ICU and hospital stay in dexmedetomidine group may be due to the lower incidence of agitation and delirium in dexmedetomidine treated group with decreased complications related to invasive mechanical ventilation. In a study that compared use of dexmedetomidine versus midazolam in patients with NIV failure [Citation20] they found that Dexmedetomidine shortened the duration of mechanical ventilation and the length of the ICU stay with lower incidence of nosocomial infections. Another study [Citation21] revealed that dexmedetomidine significantly shortened time to extubation and decreased ICU length of stay when compared to haloperidol in delirious intubated patients who were difficult to wean from mechanical ventilation mainly because of agitation and delirium. Another study [Citation22] compared early use of dexmedetomidine with placebo during NIV in patients with acute respiratory failure and found that dexmedetomidine did not improve NIV tolerance but this study was limited only to patients with acute respiratory failure and started the dexmedetomidine within 8 h of NIV and not immediately with initiation of NIV like our study without using any initial bolus dose. This study also included a small number of patients.
Bradycardia and hypotension associated with dexmedetomidine use are due to reduced sympathetic flow and activation of α2-receptors and are more common with bolus doses and high maintenance doses (>0.7 μg/kg/h) [Citation23,Citation24]. In our study more patients in dexmedetomidine group developed bradycardia compared to the baseline reading and the other two groups but none of these patients required intervention as no drop of heart rate ⩽50 beats/min. Hypotension occurred in dexmedetomidine group in the first hour after starting the drug and it was mostly in patients received bolus doses. Only dose reduction by 50% in 3 patients was done but no discontinuation of the drug was needed. Senoglu et al. [Citation25] compared dexmedetomidine and midazolam for sedation during NIV and found that dexmedetomidine causes bradycardia and hypotension which lasted for 2 h after starting dexmedetomidine. We can reduce the occurrence of bradycardia and hypotension with dexmedetomidine by slow titration of the drug and minimize the use of bolus doses.
Two patients in haloperidol group developed prolonged QTc-interval (>500 ms) (confirmed with 12 lead e c g) which improved with holding haloperidol for 2 h. The incidence of respiratory tract infection was significantly lower in dexmedetomidine group. This can be attributed to the lower incidence of endotracheal intubation and invasive mechanical ventilation and less need for supplementary sedation and analgesia with better cough and clearance of respiratory secretions.
Need for supplementary sedation and analgesia (midazolam, propofol and fentanyl) was significantly lower in dexmedetomidine group than both haloperidol and placebo groups. This lower need for supplementary sedation and analgesia with dexmedetomidine may be a contributing factor for the lower incidence of delirium and endotracheal intubation with shorter stay in ICU associated with the use of dexmedetomidine. Another study [Citation22] compared dexmedetomidine with placebo for patients with acute respiratory failure and revealed that routine early use of low dose dexmedetomidine during NIV does not improve overall tolerance of NIV with non-significant difference between the two studied groups regarding needs for supplementary sedation and analgesia. But this study included a smaller number of patients and was limited to patients with acute respiratory failure without use of initial bolus dose and slow change of infusion rate.
6 Conclusion
Dexmedetomidine is more effective than haloperidol for prevention of delirium during NIV with lower incidence of endotracheal intubation and NIV failure and lower need for supplementary sedation and analgesia. The length of ICU and hospital stay was significantly shorter in dexmedetomidine group with a lower incidence of respiratory tract infections. Bradycardia is more frequent with dexmedetomidine use.
7 Conflicts of Interest
None declared.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- M.CarronU.FreoA.S.BaHammamD.DellwegF.GuarracinoR.CosenitiComplications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trialsBr J Anaesth11062013896914
- P.GuptaM.K.PendurthiA.M.ModrykamienExtended utilization of noninvasive ventilation for acute respiratory failure and its clinical outcomesRespir Care5852013778784
- S.NavaP.CerianaCauses of failure of noninvasive mechanical ventilationRespir Care4932004295303
- American Psychiatric AssociationDiagnostic and statistical manual of mental disorders4th ed.2000American Psychiatric AssociationWashington, DC text revision
- J.JacobiG.L.FraserD.B.CoursinR.R.RikerD.FontaineE.T.WittbrodtClinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adultCrit Care Med3012002119141
- M.CharlesworthM.W.ElliottJ.D.HolmesNoninvasive positive pressure ventilation for acute respiratory failure in delirious patients: understudied, underreported, or underappreciated? A systematic review and meta-analysisLung1902012597603
- D.LongroisG.ContiJ.MantzA.FalthauserR.AantaaP.TonnerSedation in non-invasive ventilation: do we know what to do (and why?)Multidiscip Respir Med9201456
- G.HilbertB.ClouzeauH.Nam BuiF.VargasSedation during non-invasive ventilationMinerva Anestesiol7872012842846
- P.PandharipandP.A.ShintaniJ.PetersonB.T.PunG.R.WilkinsonR.S.DittusLorazepam is an independent risk factor for transitioning to delirium in intensive care unit patientsAnesthesiology104120062126
- J.A.FronteraDelirium and sedation in the ICUNeurocrit Care142011463474
- T.J.EbertJ.E.HallJ.A.BarneyT.D.UhrichM.D.ColincoThe effects of increasing plasma concentrations of dexmedetomidine in HumansAnesthesiology932000382394
- S.AkadaS.TakedaY.YoshidaA.SakamotoThe efficacy of dexmedetomidine in patients with non-invasive ventilation: a preliminary studyAnesth Analg1072008167170
- S.J.BajwaA.KulshresthaDexmedetomidine: an adjuvant making large inroads into clinical practiceAnn Med Health Sci Res32013475483
- H.L.BanhManagement of delirium in adult critically ill patients: an overviewPharm Pharmaceut Sci1542012499509
- B.D.FreemanD.J.DixonC.M.CoopersmithB.A.ZehnbauerT.G.BuchmanPharmacoepidemiology of QT-interval prolonging drug administration in critically ill patientsPharmacoepidemiol Drug Saf172008971981
- H.A.HassaballaR.A.BalkTorsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for useExpert Opin Drug Saf22003543547
- E.W.ElyR.MargolinJ.FrancisL.MayB.TrumanR.DittusEvaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)Crit Care Med29200113701379
- C.N.SesslerM.S.GosnellM.J.GrapG.M.BrophyP.V.O’NealK.A.KeaneThe richmond agitation-sedation scale: validity and reliability in adult intensive care unit patientsAm J Respir Crit Care Med166200213381344
- K.J.KalisvaartJ.F.de JongheM.J.BogaardsR.VreeswijkT.C.EgbertsB.J.BurgerHaloperidol prophylaxis for elderly hip surgery patients at risk for delirium: a randomized placebo-controlled studyJ Am Geriatr Soc53200516581666
- Z.HuangY.S.ChenZ.L.YangJ.Y.LiuDexmedetomidine versus midazolam for the sedation of patients with non-invasive ventilation failureIntern Med5117201222992305
- M.C.ReadeK.O’SullivanS.BatesD.GoldsmithW.R.AinslieBellomoDexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: a randomized open-label trialCrit Care1332009R75
- J.W.DevlinN.S.Al-QadheebChiAmyR.J.RobertsI.QawiE.GarpestadEfficacy and safety of early dexmedetomidine during noninvasive ventilation for patients with acute respiratory failure. A randomized, double-blind, placebo-controlled pilot studyChest1456201412041212
- Soo-BongYuDexmedetomidine sedation in ICUKorean J Anesthesiol6252012405411
- P.P.PandharipandeB.T.PunD.L.HerrM.MazeT.G.GirardR.MillerEffect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patientsJAMA298200726442653
- N.SenogluH.OksuzZ.DoganH.YildizH.DemirkiranH.EkerbicerSedation during noninvasive mechanical ventilation with dexmedetomidine or midazolam: a randomized, double-blind, prospective studyCurr Ther Res7132010141153