?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Acute hypertension is a common accompaniment during emergence from anesthesia especially in intracranial neurosurgical procedures and may be associated with the development of intracranial hematoma. Although various drugs have been evaluated, management of emergence hypertension in this subset of patients continues to be a challenge for anesthesiologists.
Methodology
Seventy-five patients ASA (I-II) scheduled for supratentorial craniotomy under general anesthesia were randomly allocated to one of three groups at the time of dural closure: Group “dex” received dexmedetomidine infusion in a rate of 1 μg/kg/hr, Group “labetalol” received labetalol infusion in a rate of 0.5 mg/kg/hr, and Group “control” a control group where patients received saline infusion at the same rate of dexmedetomidine and labetalol. Hypertensive episodes were managed with nitroglycerin at a dose starting from 1 μg/kg/min if systolic blood pressure exceeded 25% of its preinduction value. Hemodynamic parameters as well as the number of patients, the total dose of nitroglycerin required in each group and the time to extubation were recorded.
Results
Dexmedetomidine and labetalol had a significant effect in reducing SBP, MAP, DBP, HR during emergence from anesthesia, with more reduction of the SBP, MAP and DBP in the dexmedetomidine group and of the HR in the labetalol group in comparison with the other two groups. The number of patients needing nitroglycerin was 8 representing 32% of patients in dexmedetomidine group, 5 representing 20% of patients in labetalol group and 22 representing 88% of patients in control group (P value = 0.032). Time to emergence from anesthesia was comparable in the three groups.
Conclusion
Both dexmedetomidine and labetalol had favorable effects on hemodynamics at time of emergence from anesthesia in comparison with control group without prolongation of the time of extubation.
1 Introduction
Perioperative hemodynamic stability is of utmost importance in neurosurgical patients. An abrupt elevation of arterial blood pressure (BP) during emergence from anesthesia has been associated with many complications such as, postoperative intracranial hemorrhage (ICH) though no direct causal relation has been identified, increased intracranial pressure, and a prolonged hospital stay [Citation1].
The incidence of perioperative hypertension has been reported in various studies to be as wide as 54–91% [Citation2–Citation4]. In a study by Basali et al. it was reported to be 57% for postcraniotomy hypertension [Citation1].
Activation of the sympathetic nervous system appears to be a fundamental component of emergence hypertension, as evidenced by elevated plasma catecholamine concentrations in these patients [Citation5], and other factors have been suggested including pain, hypothermia and hypoxia [Citation6,Citation7]. Anesthesiologists have 2 options; either suppress this response if it occurred, or prevent its occurrence at the outset. Suppression of this response after release of endogenous catecholamines can be treated with anti-hypertensive drugs which are not devoid of side effects such as, an abrupt increase in intracranial pressure and a decrease in intracranial compliance, hypotension, sinus tachycardia, nausea, vomiting, sinus bradycardia, conduction delays, left ventricular dysfunction, bronchospasm, nausea and vomiting [Citation8,Citation9].
Anesthesiologists have long been using opioids, anesthetics, and antihypertensive drugs to blunt hypertensive responses in cranial surgeries at several critical moments such as intubation, pinning, incision, closure, and extubation [Citation10].
Labetalol a combined selective α1-adrenergic receptor blocker and nonselective β-adrenergic blocker given by IV bolus or continuous infusion, has been evaluated for the treatment of acute postoperative hypertension following cardiac, vascular, intracranial, and general surgeries. It has been found to be a safe and effective option, producing overall response rates of 85–100%. (8) However, its use in asthmatics, patients with heart failure and severe sinus bradycardia should be avoided. Moreover, its prolonged duration of action may mask the hemodynamic response of a possible postoperative increase in intracranial pressure that may manifest as hypertension and bradycardia [Citation2,Citation7,Citation8].
Dexmedetomidine a highly selective α2-adrenoceptor agonist with sedative, anxiolytic, analgesic and hypotensive properties, has also been evaluated by several authors as an adjuvant to anesthesia for neurosurgery with favorable perioperative hemodynamic control [Citation11–Citation15].
Although various drugs have been evaluated, management of emergence hypertension in patients undergoing cranial surgeries continues to be a challenge. In the present study we compare the effect of continuous IV infusion of dexmedetomidine and labetalol on controlling emergence hypertension in cranial surgeries for supratentorial tumors.
2 Materials & methods
2.1 Patient selection
After getting approval from the ethical committee of the anesthesia department at Cairo University and obtaining written informed consents from all patients, seventy-five patients ASA (I-II) scheduled for supratentorial craniotomy under general anesthesia were randomly allocated to one of three groups using computer software (research randomizer.org). Numbers were concealed by closed envelope.
In Group “dex” patients receive dexmedetomidine infusion at time of dural closure, in Group “labetalol” patients receive labetalol infusion at time of dural closure, and in Group “control” patients receive saline infusion at time of dural closure.
Exclusion criteria were as follows: age <18 or >65 years, ASA physical status >II, patients with impaired renal functions, uncontrolled hypertension, bronchial asthma, dysrhythmias, heart failure, Glasgow coma scale <12, the need for postoperative ventilation, history of allergy to the study drugs and surgeries lasting more than 6 hr.
2.2 Induction and maintenance of anesthesia
Before induction of anesthesia, all patients were cannulated with an 18 gauge venous cannula and premedicated with IV ranitidine (50 mg), metoclopropramide (10 mg) and dexamethasone (0.15 mg/kg), and standard monitors were applied in the form of ECG lead II (with ST segment analysis), pulse oximetry and non-invasive blood pressure. Invasive blood pressure was inserted preoperatively under local anesthesia, peripheral nerve stimulator (PNS) was used to monitor muscular relaxation and a capnogram was applied after induction of anesthesia as well as a central venous line.
Anesthesia was induced using propofol (2 mg/kg) and fentanyl (2 μg/kg), intubation was facilitated by the use of atracurium (0.5 mg/kg) and done after disappearance of second twitch of train of 4 using PNS, and lidocaine (1.5 mg/kg) was given 90 s before intubation.
Anesthesia was maintained with isoflurane (1–1.5% end tidal) in O2. Further fentanyl was given as 1 μg/kg at the time of skin incision and again at the time of bur hole.
Mechanical ventilation was adjusted to maintain an end tidal CO2 between 30 and 35 mmHg. Muscle relaxation was maintained by an infusion of atracurium (0.5 mg/kg/hr).
All patients received intraoperative fluid in the form of normal saline infusion (3 ml/kg/hr) and voluven (500 ml), and central venous pressure was maintained between 5 and 8 cm H2O. Mannitol (0.5 gm/kg) was administered shortly after induction as a brain dehydrating measure. Plasma expander was administered to maintain normovolemia and blood transfusion started if the patient hemoglobin level decreased to ⩽8 gm%.
2.3 Recovery
By the end of surgical homeostasis and start of dural closure, patients were divided randomly into 3 equal groups.
Infusions were prepared by a clinical pharmacist not included in data collection. Group “dex” received dexmedetomidine intravenous infusion in a rate of 1 μg/kg/hr prepared as 100 μ dexmedetomidine in 50 ml saline. Group “labetalol” received labetalol infusion in a rate of 0.5 mg/kg/hr prepared as 50 mg labetalol in 50 ml saline. Group “control” received normal saline infusion. The rate of the infusion in all groups was 0.5 ml/kg/hr. The attending anesthetist was blinded to the type of solution injected. Atracurium infusion was discontinued after closure of the dura, while isoflurane was discontinued after skin closure. Management of hypertension was done using nitroglycerine starting at 1 μg/kg/min if the change in systolic blood pressure exceeded 25% of its preinduction value. When hypotension (decrease in SBP ⩾ 25% from preinduction value) occurred, ephedrine 3 mg increments were given whereas atropine (0.5 mg IV bolus) was given for patients experiencing bradycardia (HR <45 beat/min).
Residual effects of muscle relaxant were reversed with neostigmine (0.05 mg/kg) and atropine 0.01 mg/kg upon appearance of second twitch of train of four. Patients were extubated upon localization to pain. The study infusions were stopped at the time of extubation.
The patients were then transferred to the PACU where all haemodynamic parameters (Heart rate, blood pressure and O2 saturation) were monitored and patients received O2 supplementation until discharge to the ward 4 hr later.
2.4 Data collected
Systolic (SBP), diastolic (DBP), mean arterial blood pressure (MAP) and heart rate (HR) were recorded at the following times: Before induction of anesthesia, before dural closure, 5 min after drug administration, every 5 min intraoperative till extubation, at the time of extubation, every 15 min for 4 hr in the PACU.
Number of patients needing nitroglycerin infusion as well as the total amount consumed by each patient was recorded, and number of patients requiring atropine and/or ephedrine was recorded as well as the total amount of ephedrine given.
Extubation time (defined as the time between the discontinuation of inhalation agents and extubation) was also measured.
2.5 Sample size
Total sample size of 75 patients randomly allocated into three equal groups. Estimation of sample size was performed using computer program G∗Power 3 for Windows (Franz Faul, Universität Kiel, Germany) with 80% power to detect a clinically significant difference of 10% or more in the SBP (effect size f = 0.424, α error = 0.05, β error = 0.2) with a result of 66 patients. We included 75 patients to guard against losses in the perioperative period.
2.6 Statistical analysis
Data were coded and entered using the statistical package SPSS version 22. Values were expressed as mean and SD for quantitative variables and frequencies (number of cases) and relative frequencies (percentages) for categorical variables. Comparisons between different groups were carried out by one way analysis of variance (ANOVA) with post hoc test.
For comparing categorical data, Chi square (χ2) test was performed. Fisher’s Exact test was used instead when the expected frequency is less than 5. P-values less than 0.05 were considered as statistically significant while P-values < 0.001 were mentioned and considered highly statistically significant.
3 Results
3.1 Demographic data and duration of surgery
There were no significant differences in demographic data between the three groups as regards patients’ age, sex, height, body weight, ASA physical status and duration of surgery ().
Table 1 Demographic data and duration of surgery.
3.2 Operative data
There was no statistically significant difference between the three groups as regards tumor site ().
Table 2 Comparison among the three groups as regards site of the lesion.
3.3 Comparison between the three groups as regards nitroglycerin and ephedrine administration
As regards ephedrine administration, there was no statistically significant difference between the three groups.
However, there was a highly statistically significant difference in the amount of nitroglycerin received between the three groups.
As regards the number of patients receiving nitroglycerin there was a statistically significant difference between the three groups. 22 patients (88%) in the control group received nitroglycerin; meanwhile, only 5 patients in labetalol group (20%) and 8 patients (32%) in dexmedetomidine group received Nitroglycerin ().
Table 3 Comparison between the three groups as regards nitroglycerin and ephedrine administration.
3.4 Comparison between the three groups as regards hemodynamics
3.4.1 A - Heart rate
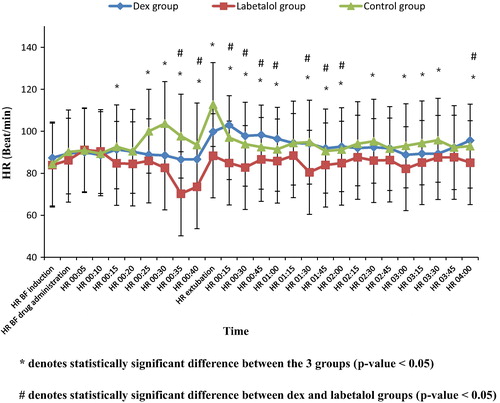
As regards the heart rate (HR), there was no statistically significant difference between the three groups in baseline readings before induction of anesthesia as well as before dural closure.
After drug administration, a statistically significant difference was observed between the three groups (p value < 0.05) at the following times, 15, 25, 30, 35, 40 min, as well as at the time of extubation where HR was less in both labetalol and dexmedetomidine groups than in the control group.
The observed statistically significant difference was high at several time intervals: HR at 30 min (dex group 88.55 ± 8.12, labetalol group 82.60 ± 11.31, control group 103.68 ± 24.31, p value < 0.001); at 35 min (dex group 86.61 ± 7.04, labetalol group 70.27 ± 15.98, control group 97.70 ± 18.71, p value < 0.001), at 40 min (dex group 86.72 ± 7.58, labetalol group 73.60 ± 9.42, control group 89.47 ± 5.94, p value < 0.001), and at the time of extubation (dex group 99.84 ± 16.48, labetalol group 88.36 ± 18.94, control 112.76 ± 18.68, p value < 0.001).
As regards the heart rate between dexmedetomidine and labetalol groups after drug administration, it was lower in labetalol group. The difference was statistically significant (P value < 0.05) at 35 and 40 min.
In the PACU, a statistically significant difference was observed between the three groups in most of the readings (p value < 0.05) where HR was lower in the labetalol group than both the dexmedetomidine and the control groups ().
The observed statistical significance was high at several time intervals: at 30 min (dex group 97.84 ± 8.94, labetalol group 82.76 ± 3.49, control group 93.88 ± 16.17, p value < 0.001), at 1:00 hr (dex group 96.44 ± 4.26, labetalol group 85.84 ± 5.37, control group 91.44 ± 13.91, P value < 0.001) at 1:30 hr (dex 94.20 ± 4.60, labetalol 80.48 ± 9.51, control 94.88 ± 17.91, p value < 0.001) (). As regards the heart rate between dexmedetomidine and labetalol groups after discontinuing the drugs, it was lower in labetalol group. The difference was statistically significant (P value < 0.05) at 45 min, and at 1:45, 2:00, 4:00 hr. It was highly statistically significant (P value < 0.001) at 15, 30 min, and at 1:00, 1:30 hr ().
3.4.2 B - Systolic blood pressure
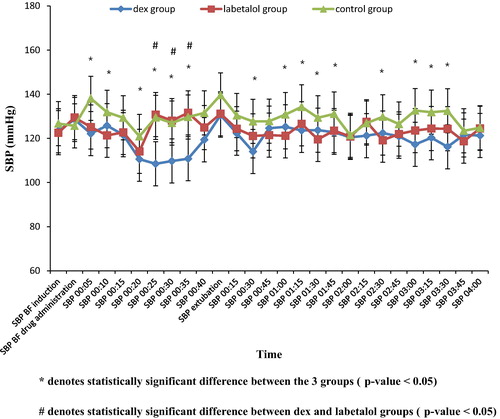
As regards SBP, there was no statistically significant difference between the three groups in baseline readings before induction of anesthesia as well as before dural closure.
After drug administration, a statistically significant difference was observed between the three groups (p value < 0.05) at the following times 5, 10, 20, 25, 30 and 35 min. SBP was lower in dexmedetomidine group than in both the labetalol and the control groups.
The observed statistical significance was high at several time intervals: at 5 min (dex group 122.16 ± 15.71, labetalol group 125.16 ± 13.92, control group 138.12 ± 13.09, P value < 0.001), at 25 min (dex group 108.48 ± 13.08, labetalol group 130.8 ± 10.92, control group 129.48 ± 23.24, P value < 0.001), at 35 min (dex group 110.78 ± 14.31, labetalol group 131.6 ± 13.47, control group 129.7 ± 8.34, P value < 0.001).
In the PACU, a statistically significant difference was observed between the three groups at several time intervals at 30 min and at 1, 1.15, 1.30, 1.45, 2.30, 3:00, 3.15, 3.30 hr (p value < 0.05). The SBP was lower in both dexmedetomidine and labetalol groups than in the control group.
The observed statistical significance was high at 30 min (dex 114.04 ± 13.84, labetalol 121.12 ± 11.3, control 127.68 ± 7.39, p value < 0.001); at 1:30 hr (dex 123.72 ± 13.2, labetalol 119.56 ± 5.66, control 129.3 6 ± 11.88, p value 0.008) and at 3:30 hr (dex 116.2 ± 15.95, labetalol 124.36 ± 12.73, control 132.48 ± 7.27, p value < 0.001).
As regards the SBP between dexmedetomidine and labetalol groups after discontinuing the drugs, there was no statistically significant difference ().
3.4.3 C - Diastolic blood pressure
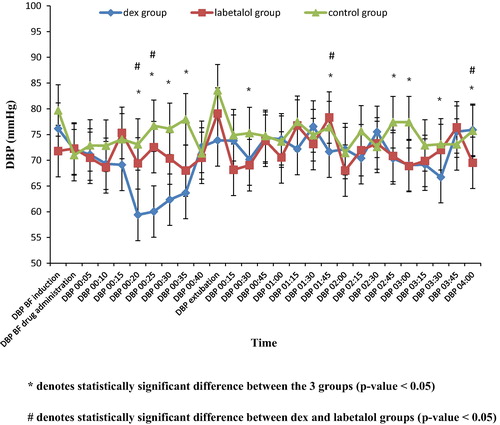
As regards DBP, there was no statistically significant difference between the three groups in baseline readings before induction of anesthesia as well as before dural closure.
Statistically significant differences were observed between the three groups (p value < 0.05) in the readings, 20, 25, 30, 35 min after drug administration. DBP was lower in the dexmedetomidine group than in both the labetalol and the control groups.
The statistically significant difference was high at 20 min (dex group 59.4 ± 5.69, labetalol group 69.4 ± 11.29, control group 73.08 ± 8.15, p value < 0.001) and at 25 min (dex group 60.04 ± 7.14, labetalol group 72.56 ± 8.29, control 76.72 ± 16, p value < 0.001).
In the PACU, there were statistically significant differences between the three groups at 30 min, 1:45, 2:45, 3:00, 3:30, and 4 hr readings.
The difference was highly statistically significant at 3 hr (dex group 69.04 ± 9.89, labetalol group 68.88 ± 5.54, control group 77.4 ± 4.25, p value < 0.001).
As regards the DBP between dexmedetomidine and labetalol groups after discontinuing the drugs, there was no statistically significant difference except at 1:45 and 4:00 hr ().
3.4.4 C - Mean blood pressure
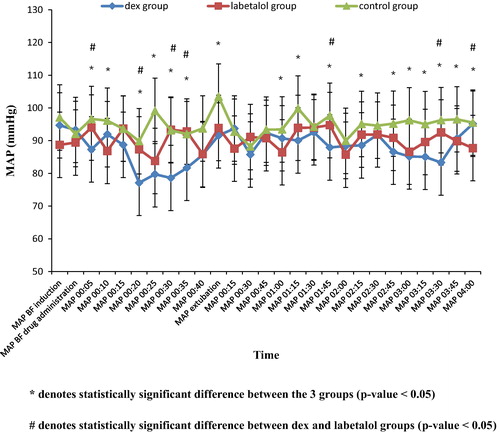
As regards MAP, there was no statistically significant difference between the three groups in baseline readings before induction of anesthesia as well as before dural closure.
Statistically significant differences between the three groups (p value < 0.05) were observed at 5, 10, 20, 25, 30 and 35 min after drug administration, as well as at the time of extubation. MAP was lower in dexmedetomidine group than in both the labetalol and the control groups.
The observed statistically significant difference was high at 5 min (dex group 87.32 ± 4.02, labetalol group 94 ± 7.72, control 96.64 ± 8.48, p value < 0.001), at 20 min (dex group 77.12 ± 5.64, labetalol group 87.36 ± 14.31, control group 89.84 ± 5.2, p value < 0.001), and at 25 min (dex group 79.74 ± 7.58, labetalol group 83.8 ± 12.88, control group 99.12 ± 19.14, p value < 0.001).
Regarding MAP between dexmedetomidine and labetalol groups after drug administration, it was lower in dexmedetomidine group in most of the readings. The difference was statistically significant (P value < 0.05) at 5, 20, 30 and 35 min.
In the PACU, there were statistically significant differences between the three groups at 1:00, 1:15, 1:45, 2:15, 2:45, 3:00, 3:15, 3:30, 3:45 and 4 hr readings.
Regarding the MAP between the dexmedetomidine and labetalol groups in the PACU after discontinuing the drugs, statistically significant differences (P value < 0.05) were only observed at 1:45 and 3:30 and 4:00 hr, where MAP was lower in dexmedetomidine group except at 4:00 hr ().
3.5 Comparison between the three groups as regards time to extubation
There was no statistically significant difference between the three groups as regards time to extubation ().
Table 4 Comparison between the three groups as regards the time to extubation.
4 Discussion
The present study showed that both dexmedetomidine in a dose of 1 μg/kg/hr and labetalol 0.5 mg/kg/hr starting at dural closure had a significant effect in reducing HR, SBP, DBP, MAP during emergence from anesthesia. The number of patients needing nitroglycerin was significantly lower in dexmedetomidine group and labetalol group in comparison with the control group. This was without prolonging the time to emergence in both dexmedetomidine and labetalol groups in comparison with control group. Moreover the HR was significantly lower in labetalol group in comparison with the dexmedetomidine group, whereas SBP, DBP and MAP were lower in dexmedetomidine group during emergence from anesthesia.
To our knowledge no comparative study between the hemodynamic effects of labetalol and dexmedetomidine on emergence from anesthesia after cranial surgeries is yet available.
The findings of the present study could be explained as follows: labetalol is a combined selective alpha-1 and non-selective beta-adrenergic receptor blocker with an alpha to beta blocking ratio of 7:1 [Citation7], with the onset of hypotensive response beginning within two to 5 min after IV administration, reaching a peak at 5–15 min, lasting for about two to 4 hr and with an elimination half life of 5.5 hr, though this variability makes labetalol very difficult to titrate as a continuous infusion [Citation7]. However, it preserves cerebral blood flow and autoregulation, and does not affect global or regional cerebral blood flow or cerebral metabolic oxygen consumption [Citation16].
Dexmedetomidine is a highly selective α2-agonist with an affinity ratio α2:α1 of 1620:1. It causes a dose dependent decrease in arterial blood pressure and HR possibly due to a decrease in serum norepinephrine concentrations. The effect of α2 agonists on hemodynamics is caused by stimulation of α2 adrenoceptors in the central nervous system [Citation17,Citation18]. It has a very short half-life of distribution, approximately 6 min, and elimination time of 2 hr [Citation19].
Furthermore, The neuroprotective effect of dexmedetomidine is thought to be due to the reduction of the levels of circulating and brain catecholamines and consequently, balancing the ratio between cerebral oxygen demand to supply, reducing excitotoxicity, and improving the perfusion in the ischemic penumbra. It reduces the levels of the glutamate responsible for cellular brain injury, especially in subarachnoid hemorrhage. It has been shown to limit the morphologic and functional effects after ischemic (focal and global) and traumatic injury to the nervous system [Citation12,Citation20].
The results of the present study were in agreement with those of the study done by Guler et al. in 2005, they studied the effect of single dose dexmedetomidine on airway and circulatory reflexes during emergence from anesthesia in sixty patients undergoing intraocular surgeries who received a standard anesthetic. Five minutes before the end of surgery, they were randomly allocated to receive either dexmedetomidine 0.5 μg/kg (n 30) or saline placebo (n 30) intravenously over 60 s. Guler et al. found that there was less significant increase in HR, SBP and DBP at extubation with dexmedetomidine with no difference in the time for tracheal extubation or for emergence from anesthesia [Citation21]. It should be noted that in our study the use of dexmedetomidine was in the form of infusion at a dose of 1 μg/kg/hr that starting at dural closure and continuing to the time of extubation.
Moreover, Bindu et al. in 2013 studied the effect of an intravenous infusion of dexmedetomidine against placebo, over 15 min before anticipated time of end of surgery. Fifty patients scheduled for elective general surgical, urological and gynecological were randomly allocated to receive either dexmedetomidine or saline, and HR, SBP, DBP and MAP were recorded at the start of injection, at 1, 3, 5, 10, 15 min till extubation, at 1, 3, 5 min after extubation, and thereafter every 5 min for 30 min. They observed a significantly higher HR, SBP, DBP and MAP in control group. This is in concurrence with our study taking into consideration the difference in surgical group, dosing and the time of start of infusion [Citation22].
Furthermore, Kothari et al. in 2014, compared the effect of dexmedetomidine versus lignocaine in attenuation of circulatory and airway responses during endotracheal extubation in craniotomies. The drugs were given 5 min before the extubation over a period of 60 s, dexmedetomidine in a dose of 0.5 μg/kg and lignocaine in a dose of 1.5 mg/kg. Kothari et al. found that Single dose of dexmedetomidine given 5 min before extubation produced significant attenuation of circulatory and airway responses as compared to Lignocaine in craniotomies which comes in agreement with our study though there is a difference in the dose used, the time and the way of administration [Citation23].
In addition, Bekker et al. in 2008 designed a study in which Patients scheduled for elective craniotomy were randomly assigned to receive either sevoflurane–opioid or sevoflurane–opioid–dexmedetomidine anesthesia. Opioids, sevoflurane, and vasoactive medications were titrated in a routine manner, by using indices, which assess a global hemodynamic stability of the anesthetics concluded that intraoperative dexmedetomidine infusion was effective for blunting the increases in SBP perioperatively, which is consistent with our study; however, there is a difference in the design between our study and Bekker’s study, in our study dexmedetomidine was given as infusion only started at the time of dural closure, while in Bekker’s study, dexmedetomidine started as loading and maintenance from start of the operation [Citation24].
Also Ilhan et al. in 2010, studied in a double-blind prospective clinical study, the effects of fentanyl and dexmedetomidine as adjuvant agents in supratentorial craniotomies on the hemodynamic changes during perioperative and recovery periods, recovery times and side effects. Thirty patients undergoing intracranial tumor surgeries were divided into two groups. In one group (n = 15), dexmedetomidine was infused as a 1 μg/kg bolus dose 10 min before induction of anesthesia and maintained with 0.4–0.5 μg/kg/min during the operation, and fentanyl was given as a 2 μg/kg at induction. In the other group (n = 15), patients were given fentanyl 4 μg/kg before induction and 0.02 μg/kg/min as an infusion for maintenance of anesthesia. Ilhan et al. concluded that dexmedetomidine controlled the hemodynamic changes better than fentanyl perioperatively, after extubation and during the early postoperative period, which supports the results of our study [Citation25].
Moreover, in a comparative study by Gosai et al. in 2015, seventy-five patients undergoing cranial surgeries were randomly divided into 3 groups to receive either 0.5 μg/kg dexmedetomidine or 1.5 mg/kg lignocaine or normal saline as intravenous boluses over one minute after return of spontaneous ventilation. They found that a single dose of intravenous dexmedetomidine administered before tracheal extubation resulted in an effective attenuation of hemodynamic responses to extubation in comparison with lignocaine [Citation26]. Those results come in accordance with the results of the present study taking into consideration the difference in the dose and the way of administration.
In a recent study, Mistry et al. in 2016 compared the effect of both dexmedetomidine and verapamil on attenuation of extubation responses in thirty patients undergoing spinal surgeries under general anesthesia. At the end of surgery just before extubation, group V received 0.1 mg/kg verapamil and group D received 0.3 μg/kg as intravenous bolus over one minute, and hemodynamic parameters including HR, SBP, DBP and MAP were recorded just before administration of the medications, 2 min afterward, after oral suction, immediately after extubation, and 1, 3, 5 and 10 min post extubation. Other parameters were duration of emergence and extubation, quality of extubation, Richmond Agitation Sedation Scale score and time to reach modified Aldrete score ⩾9. They concluded that a single dose of dexmedetomidine administered just before extubation resulted in significant attenuation of circulatory and airway responses in comparison with a single dose of verapamil [Citation27]. This comes in agreement with the present study though the study groups, the timing, the dosage and the way of administration were different.
Regarding the hemodynamic effects of labetalol, a study by Kross et al. compared the efficacy of the combination of enalaprilat/labetalol with that of enalaprilat/nicardipine to prevent emergence postcraniotomy hypertension. Forty-two patients received enalaprilat 1.25 mg IV at dural closure followed by either multidose nicardipine 2 mg IV or labetalol 5 mg IV to maintain the SBP below 140 mmHg. SBP was similarly controlled in both groups. There was a marginally smaller incidence of failures and adverse effects with labetalol. Blood pressure profiles were similar for both groups [Citation28]. That study is in agreement with the present study except that labetalol in the present study was given as a sole antihypertensive agent starting at dural closure as an infusion in a rate of 0.5 mg/kg/hr.
Congruent with our study, Muzzi et al. compared the effects of esmolol and labetalol in treating increases in blood pressure during emergence and recovery from anesthesia after intracranial surgery. Both esmolol and labetalol were equally effective in controlling systolic blood pressure on emergence and in the recovery room in patients undergoing intracranial surgery. However, decreases in heart rate were significantly more frequent in the immediate postoperative period in patients given labetalol [Citation2].
Furthermore, Sladen et al. in a multicenter study evaluated the efficacy and safety of intravenous (IV) labetalol for the control of elevated blood pressure in the intensive care unit (ICU) in 65 patients within 4 hr following coronary artery bypass grafting (CABG). Intravenous labetalol was loaded incrementally (5, 10, 20, and 40 mg at 10-min intervals) to a maximum cumulative dose of 75 mg, until either SBP decreased 10% or DBP decreased 10% and was less than 90 mmHg. Responders were entered into a 6-hr maintenance period, and received 5–40 mg of IV labetalol every 10 min as needed for blood pressure control. Hemodynamic data were recorded at baseline, just before each dose of labetalol during the loading period, and at the end of the maintenance period. Alternative therapy was given in the case of no response or adverse events. Intravenous labetalol successfully controlled post-CABG hypertension in 55 of 65 patients (85%); of these, 46 responded to 35 mg or less. Sladen concluded that IV labetalol appears to be a safe, effective agent in controlling post-CABG hypertension, with the added potential benefit of enhanced myocardial oxygen balance [Citation29]. However, Sladen et al., study differs from our study in various aspects, regarding the patient group, the method and the timing of labetalol administration; however, both studies share the same conclusion of labetalol safety and efficacy in managing postoperative hypertension.
5 Pitfalls
The lack of assessment of postoperative sedation score and the lack of assessment of the effect of the drugs on airway reflexes such as gag and cough reflexes are drawbacks of this study.
Furthermore, other studies are needed to compare the responses of the different age groups as well as controlled hypertensive patients to the study drugs, the effects of different dosages of dexmedetomidine, the infusion versus bolus doses, the different timings of the initiation of the drugs and whether dexmedetomidine can also be used for hypotensive anesthesia in surgical procedures other than neurosurgical operations.
6 Conclusion
From the present study we can conclude that dexmedetomidine infusion in a dose of 1 μg/kg/hr and labetalol infusion in a dose of 0.5 mg/kg/hr which started at dural closure had a significant effect in reducing the incidence and the extent of emergence hypertension without prolonging the time needed for extubation. There was more reduction of the SBP, MAP and DBP in the dexmedetomidine group and of the HR in the labetalol group in comparison with the other two groups.
Conflict of interest
The authors declared that there is no conflict of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- A.BasaliE.J.MaschaI.KalfasA.SchubertRelation between perioperative hypertension and intracranial hemorrhage after craniotomyAnesthesiology9320004854
- D.A.MuzziS.BlackT.J.LosassoR.F.CucchiaraLabetalol and esmolol in the control of hypertension after intra-cranial surgeryAnesth Analg7019906871
- A.F.MackenzieJ.R.ColvinG.N.KennyW.I.BissetClosed loop control of arterial hypertension following intracranial surgery using sodium nitroprusside: a comparison of intraoperative halothane or isofluraneAnaesthesia481993202204
- M.M.ToddD.S.WarnerM.D.SokollM.A.MaktabiB.J.HindmanF.L.ScammanA prospective, comparative trial of three anaesthetics for elective supratentorial craniotomyAnesthesiology199310051020
- Olsen KS, Pedersen CB, Madsen JB, Ravn LI, Schifter S. Vasoactive modulators during and after craniotomy: relation to postoperative hypertension 2002;14(3):171–9.
- H.BhagatH.H.DashP.K.BithalR.S.ChouhanM.P.PandiaPlanning for early emergence in neurosurgical patients: a randomized prospective trial of low-dose anestheticsAnesth. Analg.1074200813481355
- J.VaronP.E.MarikPerioperative hypertension managementVasc Health Risk Manag432008615627
- C.E.HaasJ.M.LeBlancAcute postoperative hypertension: a review of therapeutic optionsAm J Health Syst Pharm61200416611673 quiz 1674–5
- L.Rangel-CastillaS.GopinathC.S.RobertsonManagement of intracranial hypertensionNeurol Clin2622008521541
- K.PengS.WuH.LiuF.JiDexmedetomidine as an anesthetic adjuvant for intracranial procedures: meta-analysis of randomized controlled trialsJ Clin Neurosci21201419511958
- P.E.MarikJ.VaronPerioperative hypertension: a review of current and emerging therapeutic agentsJ Clin Anesthvol. 21 (3)2009Elsevier Inc.220229
- A.BekkerM.SturaitisDexmedetomidine for neurological surgeryOper Neurosurg572005110
- M.SturaitisJ.KroinC.SwamidossL.J.CerulloK.J.TumanEffects of intraoperative dexmedetomidine infusion on hemodynamic stability during brain tumor resectionAnesthesiology962002 A-310
- Y.GunesM.GunduzD.OzcengizH.OzbekG.IsikDexmedetomidine-remifentanil or propofol-remifentanil anesthesia in patients undergoing intracranial surgeryNeurosurg Q152005122126
- P.TanskanenJ.KyttaT.RandellR.AantaaDexmedetomidine as an anesthetic adjuvant in patients undergoing intracranial tumor surgery: a double-blind, randomized and placebo-controlled studyBr J Anaesth972006658665
- K.S.OlsenL.B.SvendsenF.S.LarsenO.B.PaulsonEffect of labetalol on cerebral blood flow, oxygen metabolism and autoregulation in healthy humansBr J Anaesth75119955154
- R.H.JamaliyaR.ChinnachamyJ.MaliwadV.P.DeshmukhB.J.ShahI.A.ChadhaThe efficacy and hemodynamic response to Dexmedetomidine as a hypotensive agent in posterior fixation surgery following traumatic spine injuryJ Anaesthesiol Clin Pharmacol3022014203207
- K.S.VoraU.BarandaV.R.ShahM.ModiG.P.ParikhB.P.ButalaThe effects of dexmedetomidine on attenuation of hemodynamic changes and there effects as adjuvant in anesthesia during laparoscopic surgeriesSaudi J Anaesth942015386392
- M.D.KarolM.MazePharmacokinetics and interaction pharmacodynamics of dexmedetomidine in humansBest Pract Res Clin Anaes142000261269
- M.KaurP.M.SinghCurrent role of dexmedetomidine in clinical anesthesia and intensive careAnesth Essays Res52011128133
- G.GulerA.AkinZ.TosunE.EskitascogluA.MizrakA.BoyaciSingle dose dexmedetomidine attenuates airway and circulatory reflexes during extubationActa Anaesthesiol Scand498200510881091
- B.BinduS.PasupuletiU.P.GowdV.GorreR.R.MurthyM.B.LaxmiA double blind, randomized, controlled trial to study the effect of dexmedetomidine on hemodynamic and recovery responses during tracheal extubationJ Anaes, Clin Pharmacol2922013162167
- D.KothariN.TandonM.SinghA.KumarAttenuation of circulatory and airway responses to endotracheal extubation in craniotomies for intracerebral space occupying lesions Dexmedetomidine versus lignocaineAnesth Essays Res8120147882
- A.BekkerM.SturaitisM.BloomM.MoricJ.GolfinosE.ParkerThe effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomyAnesth Analg1074200813401347
- O.IlhanS.KorukG.SerinI.ErkutluU.OnerDexmedetomidine in the supratentorial craniotomyEurasian J Med42220106165
- N.D.GosaiA.H.JansariR.N.SolankiD.P.PatelD.N.PrajapatiB.M.PatelA comparative study of the effect of dexmedetomidine and lignocaine on hemodynamic responses and recovery following tracheal extubation in patients undergoing intracranial surgeryInt J Basic Clin Pharmacol422015371375
- T.MistryS.PurohitG.AroraN.GillJ.SharmaAttenuation of extubation responses: comparison of prior treatment with verapamil and dexmedetomidineJ Neuroanaesthesiol Crit Care320163339
- R.A.KrossE.FerriD.LeungM.PratilaC.BroadM.VeronesiA comparative study between a calcium channel blocker (nicardipine) and a combined alpha and beta blocker (labetalol) for the control of emergence hypertension during craniotomy for tumor surgeryAnesth Analg912000904909
- R.N.SladenK.J.KlamerusM.W.SwaffordD.S.ProughH.J.MannJ.B.LeslieLabetalol for the control of elevated blood pressure following coronary artery bypass graftingJ Cardiothor Anesth421990210221