Abstract
Background
Cesarean section is considered as one of the most commonly done surgical procedures, which have a rising rate of performance. Postoperative pain may lead to poor patient satisfaction and interfere with early rehabilitation. Increasing evidence is now suggesting that less invasive regional analgesic techniques may be as beneficial as epidural analgesia. This study aimed to compare efficacy, safety and side effect of bupivacaine continuous wound infusion using constant flow PainFusor system with epidural infusion for post-cesarean section analgesia.
Methods
60 patients, ASA physical status I & II, aged 19–42 years, with full-term pregnancy undergoing elective cesarean section were randomly divided into two groups. All patients enrolled in the study performed cesarean section under standardized protocol of general anesthesia. Group A patients received continuous surgical wound infiltration, while group B patients received bupivacaine continuous epidural infusion. Pain was assessed using Visual analogue scale (VAS). Diclofenac sodium 75 mg was administered IM as a rescue analgesic.
Results
The current study showed no significant difference between the two groups in the hemodynamic parameters, respiratory parameters as well as pain scores at rest during the whole period of study. Side effects were statistically non-significant, and only patients who requested analgesia were significantly higher in group A. Furthermore, pain VAS scores on mobilization were significantly lower in group B during the first postoperative day.
Conclusion
The current study demonstrated that bupivacaine administered by continuous epidural infusion provided a significantly lower pain scores with mobilization, and hence better analgesia for post cesarean section pain in the first postoperative day compared to continuous bupivacaine wound infusion through fenestrated catheter using the constant flow PainFusor system.
1 Introduction
Effective management of postoperative pain is a patient right and essential requirement to minimize stress response following surgical intervention [Citation1]. Obstetric patients differ from other surgical patient population due to the increased concerns about postoperative pain as well as the need for early patient mobilization besides newborn care and nursing [Citation2]. Cesarean section (CS) is considered as one of the most commonly done surgical procedures, which have a progressively rising rate of performance. Being associated with intense and severe postoperative pain, CS pain may lead to poor patient satisfaction and interfere with early rehabilitation and movement in addition to improper or delayed newborn care [Citation2]. Epidural analgesia is considered as the most effective technique for postoperative pain control in abdominal surgery. Its use as an effective modality for pain relief following major surgery via local anesthetic and opioids, bolus or infusion, started as early as 1980s [Citation3]. However when using regional anesthesia, anesthesiologist may have to use certain neuraxial medications e.g. intrathecal or epidural opioids which beside improving the analgesia is associated with higher risk of side effects such as pruritus, urine retention, constipation and nausea and vomiting [Citation4]. Recently, evidence-based data suggested that the benefits of the epidural analgesia are not as significant as it was previously thought. Although it has benefits in deceasing cardiothoracic and pulmonary complications especially in high risk patients who undergo major abdominal or thoracic surgery, yet increasing evidence is now suggesting that less invasive regional techniques for analgesia e.g. paravertebral, femoral, or sciatic blocks may be as beneficial as epidural analgesia [Citation5]. Furthermore, surgical wound infusion techniques are suggested as a safe and simple alternative to epidural in various surgical procedures [Citation5].
Infiltration of the surgical wound with local anesthetic has been widely described and used for multimodal pain management [Citation6]. A systemic review of randomized controlled trials of surgical wound infiltration emphasized the safety and advantages of this technique with reduction in opioid consumption, and hence opioid side effects [Citation7]. Single bolus local anesthetic wound infiltration has been used in a wide range of surgeries including thoracic, abdominal, cardiac or pelvic procedures, yet its efficacy and duration of action were much lower than continuous surgical wound infiltration with local anesthetic through a fenestrated catheter placed above or below the muscle sheath by the surgeon at the site of surgical incision [Citation8,Citation9]. Several recommendations of well designed, large sampled, homogenous RCT were made, suggesting that such studies are valuable to optimize outcomes, and to assess the effect of continuous wound infusion on the length of hospital stay and cost effectiveness in ambulatory surgery [Citation10].
The objective of this randomized, controlled study was to compare the efficacy and side effects of bupivacaine continuous surgical wound infusion (BCWI) using a fenestrated catheter connected to a constant flow PainFusor system, with continuous epidural infusion, in controlling postoperative pain following CS in the first 24 h postoperatively. Our hypothesis was that wound infusion may offer better postoperative pain relief.
2 Patients and methods
After obtaining approval from the Clinical Research Ethics Committee of Erfan and Bagedo General Hospital and obtaining written informed consent, the study was conducted. Sixty patients, (ASA) physical status I & II, aged 19–42 years, with full-term pregnancy (37–40 weeks gestational age), body mass index ranging from 20.0 to 30.0 kg/m2, undergoing elective cesarean section were enrolled in the current study. The patients were randomly divided, using computer-conducted concealed envelope method, into two equal groups: patients in group A (n = 30) received continuous surgical wound infiltration with bupivacaine 0.25% while Group B (n = 30) patients received continuous epidural infusion with bupivacaine 0.125% and fentanyl. Inclusion criteria were ability to consent; and ability to understand and communicate (the absence of language barrier). Exclusion criteria were cardiovascular, hepatic or renal dysfunction, coagulation disorders or anticoagulant therapy, neuromuscular diseases, opioid or analgesic abuse, alcohol abuse, allergy to any of the used drugs and history of chronic pain syndrome or drug addiction.
The linear visual analogue scale (VAS) for assessment of pain, which is a 10 cm linear scale where zero is no pain and ten is the worst imaginable pain, was explained to all patients in both groups of the study before surgery starts. Patients were asked to locate their pain on this linear scale. All surgical procedures were performed by the same surgeon. All patients after admission to operating theater were placed in sitting position; the back was sterilized, and local anesthesia given. Then an 18 gauge Tuohy needle was used to insert epidural catheter using loss of resistance to air technique through midline approach at the level of L3-4 vertebrae. 10 ml of normal saline was injected to ensure catheter patency. All patients enrolled in the study performed CS under standardized protocol of general anesthesia, where only ondansetron 0.1 mg/kg was given as the sole premedication and induction was done using propofol 2 mg/kg and succinylcholine 1 mg/kg followed by atracurium 0.5 mg/kg, and maintained using sevoflurane with 50% nitrous oxide in oxygen, and after delivery of the baby fentanyl 100 μg was given. In both groups, after closure of the peritoneum the surgeon was asked to puncture the skin 2–3 cm lateral to the end of the skin incision and pass a 15 cm long fenestrated catheter (Baxter PAINfusor® catheter 15; Baxter Healthcare S.A., Zurich, Switzerland) placing it pre-peritoneal under the fascia such that the fenestrations are available along the whole wound length. The catheter was then taped and fixed to the skin to prevent its slipping out or changing position during the rest of the surgical procedure. 10 ml normal saline was injected through the catheter to confirm its patency.
Following the end of surgery, and immediately after patient extubation, in group A: bupivacaine 0.25% infusion was started at a rate of 10 ml/h in the abdominal catheter using the constant infusing PainFusor system (Baxter LV5 INfusor® and PAINfusor; Baxter Healthcare International Inc, Deerfield, IL 60015 USA) and epidural infusion was started with normal saline as a placebo at the same rate as in the other group. On the other hand in group B: normal saline was connected to the catheter as a placebo simulating group A and epidural infusion of bupivacaine 0.125% with fentanyl 2 μg/ml was started at a rate of 10 ml/h. Infusions were continued for 24 h postoperatively. In both groups, patients were given 1 g Paracetamol every 12 h intravenously in the first 48 postoperative hours. In case of breakthrough pain, if the VAS recorded increased more than 5, Diclofenac sodium 75 mg was administered intramuscular (IM) as a rescue analgesic. Moreover, in case of: bradycardia less than 50 BPM, oxygen saturation less than 90%, bradypnea less than 8 breath/min, sedation ⩾2, or hypotension less than 90/60 mmHg the infusions were stopped and these parameters were assessed every five minutes till they return to normal and then the infusions were restarted. Patients were monitored all through the study. All medications and intravenous solutions were prepared in the pharmacy outside the operating theater and sent to the anesthesiologist as apparently identical infusions with a predetermined rate; in addition, patients as well as the anesthesiologist and the surgeon were blinded to the type of the medications infused and the master codes were kept with a person that does not share in the collection or analysis of the results.
The Primary and secondary outcomes were assessed at 1, 6, 12, and 24 h postoperatively and then a follow-up reading at 48 h was recorded. Primary outcome variables of the study were the presence of VAS > 5 indicating unbearable pain at rest or on mobilization (whether coughing or ambulation), during the first 24 h after surgery, whereas secondary outcome variables included hemodynamic parameters (namely systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR)), respiratory parameters (namely respiratory rate ‘RR’ and oxygen saturation ‘Spo2’), number of patients requested rescue analgesic, in addition to incidence and degree of sedation as evaluated by a 5-point rating scale such that 0 is fully awake, 1 is drowsy but responding to verbal commands, 2 is sedated but easily aroused by light touch, 3 is sleepy but aroused by painful stimulus, and 4 is deeply sedated, not arousable. Other complications including incidence of postoperative nausea and vomiting (PONV) and the need for antiemetic medications, pruritus, hypotension, bradycardia, low oxygen saturation as well as urine retention were also recorded.
Data were analyzed using computer statistical software system SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Descriptive data were expressed as mean (±SD) or number (proportion) as appropriate. Patient demographic data and rescue analgesia administered were analyzed and compared using Student’s t-test and Fisher’s exact test, whereas differences between the groups in pain scores were analyzed and compared using Mann-Whitney U test, and Bonferroni correction was performed because of the presence of repeated measures. Sample size was calculated using Power Analysis and Sample Size 14.0 software PASS (NCSS, Kaysville, UT, USA). It was estimated using pain scores as the primary variable. Assuming pain score difference of 2 ± 2 standard deviation (SD), a priori sample size analysis was done and calculated to be 56 patients (28 per group) would be sufficient to detect a difference on the VAS with α = 0.05 (two tailed) and 90% power. However, 30 patients were enrolled in each group to ensure adequate power and allow for possible drop-outs. The incidence of complications as pruritus, PONV, urine retention was analyzed and compared using Fisher’s exact test. P-value less than 0.05 was considered statistically significant except for pain scores when Bonferroni correction was used, where a P-value less than 0.01 was considered to be statistically significant (0.05 divided by 5 repeated measures).
3 Results
60 patients were assessed for eligibility for the current study all of which were enrolled in the study and were randomly classified into two equal groups. All patients of group B succeeded to complete the study, while in group A one patient failed to complete the study because the catheter was blocked after 8 h postoperatively and was excluded from the study (). The current study showed no differences in demographic data including age, body weight or body mass index (BMI) between group A and group B as seen in . The mean duration of the CS procedure was comparable in both groups ().
Table 1 Demographic characteristics and operative data of patients receiving bupivacaine wound infusion (group A) and bupivacaine epidural analgesia (group B). Values are expressed as mean (SD).
Similar to demographic data, the hemodynamic parameters such as systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) in the current study showed no significant difference in the values measured among both groups as shown in . Furthermore, no significant difference was detected between the groups regarding respiratory parameters namely respiratory rate (16.4 ± 2.0 breath/min) for group A versus (17.1 ± 1.8 breath/min) for group B (P = 0.16), and oxygen saturation of 98.23 ± 0.1 versus 98.30 ± 0.3 for groups A and B respectively (P = 0.23).
Table 2 Postoperative hemodynamic parameters (secondary outcome) for both bupivacaine continuous wound infusion (group A) and bupivacaine epidural analgesia (group B) following cesarean section. Values expressed as mean (SD).
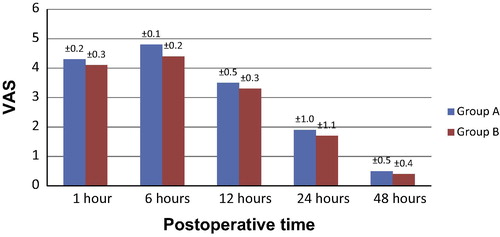
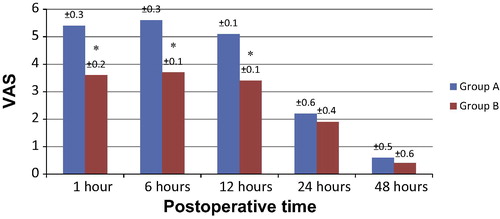
Regarding pain scores as evaluated by VAS, no significant difference was detected between the two groups in pain scores at rest during the whole period of study at 1, 6, 12, or 24 h (). However, at 6 h the VAS scores on mobilization were significantly lower in group B compared to group A (P < 0.001) with a confidence interval (95% CI 0.1–0.2) as shown in . Similarly, at 1 and 12 h VAS scores were significantly lower in group B, yet the recorded values in group A decreased to show no statistically significant difference from group B at 24 (). Regarding the follow-up after 48 h, no significant difference was detected between the two groups both at rest and mobilization ( and ).
As for the other secondary outcome variables, five patients in group A requested rescue analgesia as shown in and were given Diclofenac which was significantly higher than in group B where no patients requested analgesia (P = 0.02) with a confidence interval (95% CI 0.08–0.35). On the contrary, seven patients in group B suffered from pruritus which were significantly higher than in group A which showed no pruritus in any of the group patients (P = 0.006) with an absolute risk reduction of 0.23 (95% CI 0.8–0.38) (). Only one patient in group B showed PONV not necessitating antiemetic medication, as well as other patient in the same group developed temporary hypotension which was managed by bolus IV 500 ml of normal saline solution. Furthermore, one patient in group B developed urine retention necessitating temporary catheter insertion. None of the patient of the current study in both groups developed sedation, bradycardia or low oxygen saturation with no significant difference among the two groups in these secondary variables as demonstrated in .
Table 3 Secondary outcome variables and complications of patients with bupivacaine continuous wound infusion (group A) and bupivacaine epidural analgesia (group B) following cesarean section. Values are expressed as number (proportion).
4 Discussion
The results of the present prospective randomized placebo-controlled study concluded that continuous epidural analgesia provided lower pain scores indicating superior control of pain only during patient mobilization in the first postoperative day following cesarean section more than continuous surgical wound infusion with local anesthetic using continuous constant PainFusor system. On the other hand, pain at rest, showed no significant difference between the groups during the first postoperative day. Follow-up pain scores, at rest or mobilization, during the second postoperative day were comparable. Furthermore, rescue analgesia requirement was significantly higher during the first day postoperatively in continuous surgical wound infusion group. However, fewer complications were seen in bupivacaine wound infusion group compared to bupivacaine continuous epidural infusion which showed a significantly higher incidence of pruritus, as well as incidence of PONV, urine retention and hypotension. Besides, no patients in both groups were complicated with postoperative sedation or bradycardia, and the hemodynamic parameters such as HR, SBP and DBP as well as respiratory parameters RR and Spo2 also showed no significant changes in both groups. Such results suggested that continuous surgical wound infusion though may not offer a superior alternative to continuous epidural infusion with local anesthetic for pain control in the first postoperative day after elective cesarean section during mobilization, yet it offered comparable pain relief at rest.
Consisting of several different components, postoperative pain associated with cesarean section operation arises mainly from a visceral component from the uterus and a somatic parietal component from the muscle and cutaneous nociceptors [Citation11]. For that reason, assumption was done that the placement of fenestrated catheter in such position improves pain control being close to the peritoneum and muscle fascia which are rich in pain nerve endings damaged by the surgical incision [Citation12]. However, several concerns still arouse about the ideal protocol for using continuous surgical wound infusion for management of postoperative pain, including the best surgical plane for catheter application, the type and concentration of the local anesthetic used, the length of the catheter and number of fenestrations, and the flow rate of the local anesthetic whether constant or changeable [Citation13]. Many clinical trials were done to identify the best location for the catheter suggesting various positions including, above the fascia [Citation14,Citation15], intraperitoneal [Citation16], as well as above and below the fascia [Citation17]. These studies generally suggested a decrease in the opioid consumption but with no difference in the overall pain scores at rest or mobility compared to the control groups. Our choice for the pre-peritoneal location of the catheter was based on previous studies [Citation11,Citation18,Citation19] that showed better pain score.
The efficacy, safety and side effects of surgical wound infiltration with local anesthetic for postoperative pain relief following cesarean section were evaluated in previous studies [Citation19,Citation20]. In a study by Ranta et al. [Citation20] the author compared bupivacaine surgical wound infiltration to epidural analgesia using repeated bolus doses of local anesthetic in both routes. They concluded that the incisional infiltration with local anesthetic using a sub-fascial catheter provided analgesia comparable to epidural analgesia, yet in the first four postoperative hours, their study results showed better pain scores with the epidural analgesia group which supports the results of our study. On the contrary to the present study, in a later study done by O’Niell and colleagues [Citation19] comparing continuous wound infusion with ropivacaine for controlling postoperative pain following cesarean section with epidural morphine bolus injections, their conclusion was that wound infusion was easy and safe and provided better analgesia than epidural morphine with lower side effects besides being a good choice if epidural was not possible. We suggest that the conflict between their pain scores and the current study results can be explained by the use of continuous epidural infusion in our study instead of repeated bolus administration, besides the use of epidural combination of local anesthetic and opioid. Nevertheless, in a letter to the editor, Bhatia and Sen [Citation21] considered that a great limitation of that study was the use of single doses, both ropivacaine and morphine, in both groups suggesting a better trial with different drug doses for best dose identification. Furthermore, the result of the study by Zohar et al. [Citation14] which compared surgical wound infiltration using patient controlled analgesia (PCA) with systemic non-steroidal anti-inflammatory drugs (NSAID) for post-cesarean section pain, showed inferior analgesia and lower patient satisfaction in the wound infiltration group. The author emphasized the importance of the systemic use of adjuvant NSAID for postoperative pain management, a result that was consistent with the recorded pain scores in the current study. Moreover, in a study by Dowidar and colleagues [Citation22] who compared postoperative analgesia using continuous wound infusion with that using rectus sheath catheter, they concluded that ultrasound guided rectus sheath catheter provided superior analgesia with a significantly lower VAS, heart rate and blood pressure compared to continuous surgical wound infusion. Going with the present study, the VAS in their study was significantly higher with movement in the continuous wound infusion group. In addition, Kainu et al. [Citation23] concluded in their study that ropivacaine continuous wound infusion fails to decrease the oxycodone PCA as well as pain scores compared to intrathecal morphine in post-cesarean section pain management.
There are some limitations for our current study including the use of plain bupivacaine continuous wound infusion, whereas using additives e.g. opioids may result in better analgesic outcome. In addition, the use of a changing flow PainFusor system might have resulted in better pain scores. Moreover, another limitation to the current study was that the outcome analgesia in wound infusion technique is dependent on proper catheter position with the fenestration available along the whole length of the wound. Furthermore, the undetermined optimal local anesthetic concentration, type and flow rate are also considered as further limitations for the study.
In conclusion, although continuous surgical wound infusion with local anesthetic is a safe, easy and simple technique for postoperative analgesia, yet continuous epidural analgesia provided superior pain control during mobilization in the first postoperative day. The present study demonstrated that continuous epidural infusion with bupivacaine 0.125% and fentanyl 2 μ/ml at a rate of 10 ml/h provided a significantly better analgesia and lower pain score for post cesarean section pain during mobilization in the first postoperative day compared to bupivacaine 0.25% continuous wound infusion at rate of 10 ml/h through fenestrated catheter using the constant flow PainFusor system. However, pain relief at rest was comparable. Moreover, higher incidence of side effects was reported with epidural analgesia rather than wound infusion.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgment
The author would like to thank Erfan & Bagedo General Hospital for financing the current study.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- H.KehletSurgical stress: the role of pain and analgesiaBr J Anaesth631989189195
- B.CarvalhoS.E.CohenS.S.LipmanPatient preferences for anaesthesia outcomes associated with cesarean deliveryAnaesth Analg101200511821187
- R.G.WheatleyS.A.SchugD.WatsonSafety and efficacy of postoperative epidural analgesiaBr J Anesth87120014761
- A.MacarioM.WeingerP.TruongM.LeeWhich clinical anaesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anaesthesiologistsAnaesth Analg88199910851091
- N.RawalEpidural technique for postoperative pain: gold standard no more?Reg. Anesth. Pain Med.3732012310317
- H.KehletJ.DahlAnaesthesia, surgery, and challenges in postoperative recoveryLancet362200319211928
- S.S.LiuJ.M.RichmanR.C.ThirlbyC.L.WuEfficacy of continuous wound catheters delivering local anaesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trialsJ Am Coll Surg2032006914932
- P.WhiteN.RawalP.LathamUse of a continuous local anaesthetic infusion for pain management after medial sternotomyAnaesthesiology992003918923
- E.ForastiereM.SofraD.GiannarelliEffectiveness of continuous wound infusion of 0.5% ropivacaine by On-Q pain relief system for postoperative pain management after open nephrectomyBr J Anaesth1012008841847
- R.ShaneH.CeciliaF.MalinRopivacaine for continuous wound infusion for postoperative pain management: a systemic review and meta-analysis of randomized controlled trialsEur Surg Res5320144360
- P.Lavand’hommeF.RoelantsH.WaterloosM.De KockPostoperative analgesic effects of continuous wound infiltration with diclofenac after elective cesarean deliveryAnaesthesiology106200712201225
- S.PelesA.MirandaR.ShakerJ.SenguptaAcute nociceptive somatic stimulus sensitizes neurones in the spinal cord to colonic distension in the ratJ Physiol5602004291302
- H.KehletB.KristensenLocal anaesthetics in the surgical wound: is the pendulum swinging toward increasing useRegional Anaesth Pain Med342009389390
- E.ZoharA.ShapiroA.EidinovPostcesarean analgesia: the efficacy of bupivacaine wound instillation with and without supplemental diclofenacJ Clin Anaesth182006415421
- B.FredmanA.ShapiroE.ZoharThe analgesic efficacy at patient-controlled ropivacaine instillation after cesarean deliveryAnaesth Analg91200014361440
- A.GuptaP.PerniolaK.AxelssonPostoperative pain after abdominal hysterectomy: a double blinded comparison between placebo and local anaesthetic infused intraperitoneallyAnaeth Analg99200411731179
- T.RackelboomP.PasquierF.GoffinetAnalgesic infiltrations after cesarean section: which site of administration and cost effectiveness?Anaesthesiology1092008A1121
- M.BeaussierH.El’AyoubiE.SchifferContinuous below the fascia infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgeryAnaesthesiology1072007461468
- P.O’NiellF.DuarteI.RibieroRopivacaine continuous wound infusion versus epidural morphine for postoperative analgesia after cesarean delivery: a randomized controlled trialAnaesth Analg1142012179185
- P.O.RantaT.I.Ala-KokkoJ.E.KukkonenIncisional and epidural analgesia after cesarean delivery; a prospective, placebo-controlled, randomized clinical studyInt J Obstet Anesth1532006189194
- N.BhatiaI.SenRole of adjuvants in continuous wound infusion analgesia for cesarean deliveryAnaesth Analg11532012735
- A.M.DowidarH.A.EzzA.A.ShamaPostoperative analgesia of ultrasound guided rectus sheath catheters versus continuous wound catheters for colorectal surgery: a randomized clinical trialEgypt J Anesth3232016375383
- J.P.KainuJ.SarvelaP.HalonenContinuous wound infusion with ropivacaine fails to provide adequate analgesia after cesarean sectionInt J Obstet Anesth2122012119124