Abstract
Purpose
Adding novel drugs like pregabalin to analgesic regimens might reduce postoperative pain, total opioid consumption and side effects, this study compares multiple doses of pregabalin for postoperative analgesia following radical cystectomy.
Methods
This study is registered at http://www.clinicaltrials.gov at no.: NCT02724293. Sixty patients were randomized into 4 groups: Group I: control (placebo) group, Group II: received pregabalin 300 mg 2 h preoperatively, Group III: received pregabalin 300 mg 2 h preoperatively and 12 h thereafter, Group IV: received pregabalin 600 mg 2 h preoperatively. Postoperative pain, time to first request of analgesia, and total morphine consumption were recorded.
Results
VAS was significantly reduced in groups II, III, IV in comparison with group I immediately postoperative, and after 2 h (P < 0.05). Sedation score was significantly higher in groups II, III, IV compared to group I immediately postoperative (P < 0.05). First request of analgesia was significantly delayed in groups II, III, IV compared to control group (P = 0.000). Total analgesic consumption was significantly reduced in groups II, III, IV compared to group I (P = 0.000). Group IV showed a significantly higher incidence of dizziness compared to group I.
Conclusion
Peri-operative pregabalin at doses of 300 mg and 600 mg reduced postoperative opioid consumption and prolonged time to first request of analgesia in patients who underwent radical cystectomy, and a single preoperative dose of 600 mg is superior in analgesia to others, without serious side effects.
1 Introduction
Numerous studies have explored undesirable effects of unrelieved pain with maximum effects on different body systems. These effects include adrenal sympathetic hyperactivity, myocardial ischemia, deep venous thrombosis, difficulty of breathing, atelectasis, tachycardia, hypertension, and others [Citation1].
Opioids represent the cornerstone in postoperative pain management despite serious side-effects [Citation2] that might impair patient recovery after surgery [Citation3].
Multimodal postoperative analgesic regimens may decrease the incidence of complications, shorten the requirement for hospitalization, and decrease recovery times and health costs [Citation4].
Pregabalin is a structural analogue of the inhibitory neurotransmitter gaba-aminobutyric acid, with anticonvulsant, anti-hyperalgesic, and anxiolytic properties such as gabapentin, but with a more favorable pharmacokinetic profile [Citation5,Citation6]. There are several reports for the use of pregabalin in the management of postoperative pain with a positive result in a variety of surgical models [Citation7–Citation9].
Thus, using novel adjuvant drugs such as pregabalin, as a part of a multimodal analgesic regimen, might be reasonable for lowering postoperative pain scores, decreasing total opioid consumption, and hence, side effects [Citation10,Citation11].
Till now, there is no agreement upon the ideal dose of pregabalin when used as an adjuvant to a multimodal analgesic protocol for postoperative analgesia following major surgery. This randomized, double-blinded, controlled study was designed to examine the analgesic efficacy of three different pre/peri-operative doses of pregabalin following radical cystectomy and urinary diversion in comparison with placebo, in search of the ideal dosage.
2 Patients and methods
This prospective, randomized, double-blinded, controlled study was started after Institutional Ethics Committee approval (South Egypt Cancer Institute – Assiut University) and after obtaining written informed consent from all participating patients. It is registered at http://www.clinicaltrials.gov at no.: NCT02724293. The study was conducted according to the most recent version of the Declaration of Helsinki. Sixty patients between the ages of 18 and 60 years with ASA I-II physical status who underwent radical cystectomy under general anesthesia were enrolled in this study. Patients were randomized into 4 groups (15 patients in each):
Group I: control group (placebo group).
Group II: patients received pregabalin 300 mg 2 h preoperatively.
Group III: patients received pregabalin 300 mg 2 h preoperatively and 12 h after the preoperative dose.
Group IV: patients received pregabalin 600 mg 2 h preoperatively.
Randomization was done using lottery method. Pregabalin was given orally by a staff nurse who was not included in the study. Anesthesiologists and patients were blinded to the groups.
2.1 Exclusion criteria
Patients with a history of drug or alcohol abuse and patients with chronic pain or daily intake of analgesics, uncontrolled diabetes mellitus and/or hypertension, atherosclerotic heart disease, seizures, impaired kidney or liver functions, body mass index ⩾35 kg/m2, and who could not control a patient-controlled analgesia (PCA) device were excluded from the study.
2.2 Anesthetic management and operation
One day before surgery, patients were trained on how to use the PCA pump that when they feel pain, a push to the button will relieve it, but they cannot push the button frequently to avoid overdose (lock out period). They were also taught how to express the level of pain they experience using an 11-point Visual Analogue Scale (VAS), with 0 indicating no pain and 10 indicating the worst imaginable pain. On arrival to the operating room, an intravenous line was inserted. Patients were pre-medicated with 0.25 mg/kg intravenous ranitidine. Monitoring included electrocardiography, noninvasive blood pressure (NIBP), O2 saturation, and temperature. Anesthesia was induced for all participating patients with 1.5–2 μg/kg fentanyl, 1–2.5 mg/kg propofol, and 1.5 mg/kg lidocaine. Endotracheal intubation was facilitated by 0.15 mg/kg cis-atracurium. Anesthesia and muscle relaxation were maintained by 1–1.5 MAC isoflurane in 50% oxygen/air mixture and 0.03 mg/kg cisatracurium, respectively, and mechanical ventilation was maintained in parameters that keep ETCo2 in the range of 35–40 mmHg. Intravenous crystalloid solution was infused at a rate of 8 mL/kg/h to correct for third space loss apart from added losses, and blood transfusion was allowed when hemoglobin is <10 g/dl, or when hematocrit value is <30%.
2.3 Patient controlled analgesia and pain scores
At the end of surgery, residual neuromuscular paralysis was antagonized with neostigmine 0.04 mg/kg and atropine 0.02 mg/kg. The patients were connected to a morphine patient controlled analgesia (PCA) pump (Perfusor® Space PCA Infusion Pump System, B. Braun, USA) on arrival at the PACU. The PCA pump was set to deliver a loading dose of 2.5 mg and an incremental dose of 2.5 mg at a lockout interval of eight minutes and a four-hour limit of 50 mg. Sedation level was evaluated using a 4-point sedation scale where 0 = awake, 1 = easily aroused, 2 = awakens after tactile stimulation, 3 = awakens after verbal stimulation, and 4 = not arousable [Citation12]. Vital signs, visual analogue scale (VAS), total morphine consumption and adverse effects such as nausea, vomiting, pruritus, headache, dizziness, and visual abnormalities (double or blurred) were recorded.
Our primary outcome measure was the efficacy of the studied doses in reducing postoperative total analgesic consumption. Secondary outcome measures included reduction of postoperative pain scores, time to first request of rescue analgesia, and the tolerability of the used doses represented by the side effects during the follow-up period of 24 h.
3 Statistical analysis
3.1 Power of the study
The primary end point was the total dose of intravenous PCA morphine consumption in the first 24 h postoperatively. Secondary endpoints were the safety profile of the studied doses in terms of predefined adverse events, nausea, vomiting, and level of sedation during the study period. A calculated sample size of 12 patients in each group would have an 80% power of detecting a difference of 20% decrease in intravenous PCA morphine consumption at a 0.05 level of significance using a confidence interval of 95%. We enrolled 15 patients in each group to compensate for possible dropouts.
3.2 Data analysis
Analysis was performed using statistical package for the Social Sciences software, ver. 20 (SPSS Inc., Chicago IL, USA). Data were presented as mean ± SD, numbers, and percentages. Mann-Whitney was used to compare between each two groups. Chi-square test was used for comparison between percentages and frequencies. P < 0.05 was considered significant.
4 Results
This study was conducted on 60 patients who underwent radical cystectomy for management of urinary bladder cancer. Patients were given different doses of perioperative pregabalin in order to investigate its analgesic efficacy and safety.
Regarding the demographic and clinical data of the participating patients, there was no significant difference between group I (control group) and the study groups II, III, IV .
Table 1 Personal and clinical data.
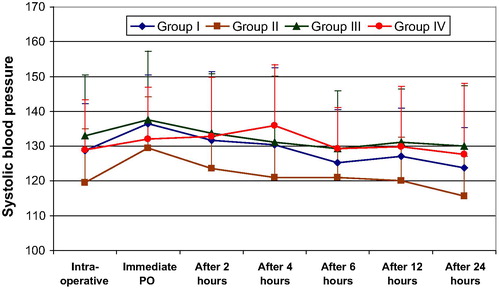
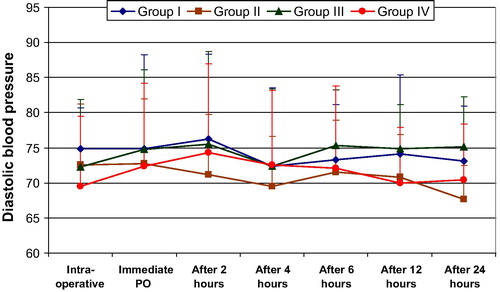
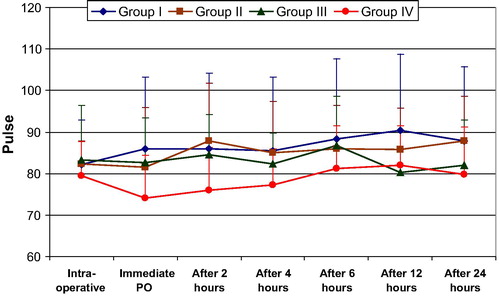
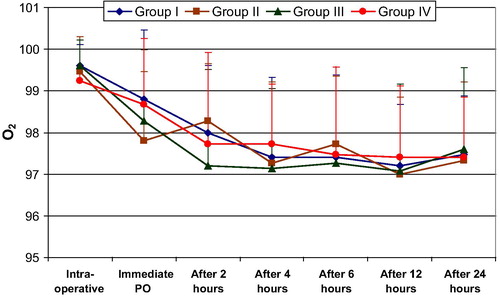
Looking into the hemodynamic changes and changes in arterial oxygen concentration in the intra-, and post-operative periods, there was no significant difference between study groups II, III, IV, and the control group I .
VAS showed a significant reduction in groups II, III, IV in comparison with control group I immediately postoperative, and after 2 h (P < 0.05). After that time, VAS values did not significantly differ between the four study groups till the end of the 24 h of observation .
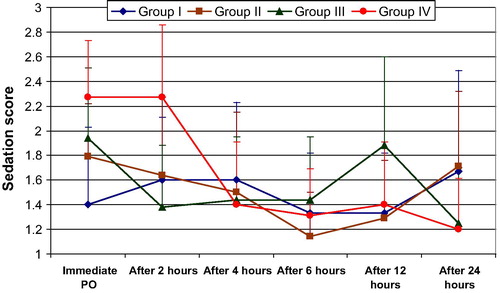
Sedation score was significantly higher in groups II, III, IV in comparison with the control group I immediately postoperative (P < 0.05). Two hours postoperatively, only group IV (600 mg pregabalin) continued to show significantly higher sedation score compared to control group I (P = 0.005). After 12 h postoperatively, only group III (300 mg pregabalin twice) showed a significantly higher sedation score compared to control group (P = 0.028). .
First request of rescue analgesic was significantly delayed in groups II, III, IV in comparison with control group (P = 0.000) . First request of analgesia was also significantly delayed in groups III and IV compared to group II, and in group IV compared to group III (P = 0.000).
Table 2 First request of analgesia and total analgesic consumption.
Total analgesic consumption was significantly reduced in groups II, III, IV in comparison with group I (P = 0.000). . On comparing groups II, III and IV, there was no significant difference between them (P > 0.05).
Considering the side effects experienced by patients in different groups during the study, a significantly lower number of patients had vomiting in group IV (0 patients) compared to group I (6 patients) (P = 0.022). On the other hand, group IV showed a significantly higher incidence of dizziness (7 patients) compared to the groups I and II (1 patient) (P = 0.039) .
Table 3 Side effects.
5 Discussion
This double-blinded, prospective, controlled trial investigated the efficacy and safety of three different doses of pre/peri-operative pregabalin for postoperative pain management in patients who underwent radical cystectomy.
We found that there was a significant reduction of postoperative VAS score (in the first two hours), total rescue analgesic consumption, and delay of first request of recue analgesia in all the study groups compared to the control group. Sedation was significantly higher in all groups compared to control.
Prevention and treatment of postoperative pain continues to be a major challenge in the postoperative period upon which early mobilization and well-being of the surgical patients depend. Gabapentinoids are recently used for postoperative analgesia. Pregabalin is claimed to be more effective in preventing neuropathic component of acute postoperative pain, and to produce more opioid sparing effect than gabapentin.
The probable mechanism of action of pregabalin is through potent binding at α2-d subunit of the presynaptic, voltage-gated calcium channels that are widely distributed throughout the peripheral and central nervous systems [Citation11,Citation13], and this reduces calcium influx and therefore reduces the release of several neurotransmitters at nerve terminals including glutamate, norepinephrine and substance P [Citation14,Citation15]. Also, it reduces the hyperexcitability of dorsal horn neurons, the sensitization of which is a component in acute pain models [Citation16,Citation17]; thus, pregabalin may have a role in postoperative pain management [Citation18,Citation19].
So far, analgesic properties of pregabalin have been tested only in controlled randomized trials conducted in patients of dental pain, minor and day-case gynecological surgery, laparoscopic hysterectomy and hip arthroplasty [Citation20–Citation23].
As most of the previous studies found that the peri-operative use of pregabalin at doses less than 300 mg had no analgesic efficacy, and that it did not have analgesic sparing effects, we used higher doses. A dose of 600 mg given 2 h before surgery is not exceeding the maximal daily dose of pregabalin, and it is expected to be more effective in the management of postoperative pain after a major surgery like radical cystectomy; however, side effects need to be explored, especially in the first 24 h of postoperative period.
VAS values were significantly lower in all pregabalin groups II, III, and IV compared to control group I in the first two hours of the postoperative period. Moreover, there was a reduction in total rescue analgesic (morphine) consumption in the three study groups compared to control group. There was a significant delay of first analgesic request in the three study groups compared to control group and it was significantly delayed in group IV compared to group III; this is most probably the effect of the large single dose (600 mg) given to patients in group IV preoperatively; however, this was not translated into a significant change in the total amount of analgesic given to patients in these two groups (groups III and IV with the larger pregabalin dose among the four study groups), and this could be explained by the second dose of pregabalin of 300 mg given to patients in group III after 12 h from the initial dose which, in our opinion, enforced its analgesic efficacy and reduced the need to rescue analgesia.
These findings are compatible with most of the studies carried out in this respect, where, Hill and co-workers [Citation24] found 300 mg pregabalin to be more effective than 50 mg pregabalin or 400 mg ibuprofen in attenuating pain after dental extraction. Moreover, in a study done by Ittichaikulthol and colleagues [Citation25], they found that 300 mg 1 h before surgery, significantly reduced pain scores and morphine consumption after abdominal hysterectomy. Mathiesen et al. [Citation23] concluded that Pregabalin resulted in a 50% reduction in 24 h postoperative morphine requirements, but it was associated with a higher level of sedation compared to placebo. Likewise, Jokela et al. [Citation21] found that peri-operative use of pregabalin 600 mg (300 mg one hour before surgery, and 12 h after first dose) was associated with reduction of postoperative oxycodone consumption. Remarkably, Akhavan-akbari et al., confirmed that even a single pre-operative oral dose of pregabalin 150 mg is an effective method for reducing postoperative pain and pethidine consumption in patients undergoing orthopedic surgery [Citation26].
Sedation scores were significantly higher for the three groups immediately postoperative in comparison with control group, and in group IV it continued to be significantly higher in the first 2 h. This is mostly due to the larger dose of pregabalin used at this group. Another increase at the sedation score was noticed after 12 h postoperatively in group III in comparison with control, which could be attributed to the second dose of 300 mg pregabalin given to patients in this group after 12 h from the first dose (nearly 2 h before this measurement of sedation score).
Nausea and/or vomiting occurred in 6 patients in group I most likely due to the effects of higher dose morphine used as a rescue analgesic in this group in comparison with others, and dizziness was of higher incidence in group IV (7 patients) in comparison with group I (only 1 patient). Somnolence and dizziness are the two most common side effects associated with gabapentin and pregabalin [Citation27]. This is usually not disabling and antianxiety effect has been found to be beneficial in some studies [Citation28].
This agrees with the findings of Jokela and colleagues, where the degree of drowsiness was similar after perioperative administration of diazepam 10 mg, pregabalin 300 mg, or 600 mg following laparoscopic hysterectomy. The incidence of dizziness and blurred vision was higher after perioperative administration of pregabalin 600 mg during the first 24 h after surgery [Citation21].
In a study by Ghai and colleagues, 600 mg pregabalin was associated with excess somnolence up to 18–24 h after surgery. Further cases were abandoned with 600 mg though most of these cases did not require any analgesic in the first 24 h [Citation29].
We believe that our work is limited by the small number of participating patients, and the relatively short follow-up period of 24 h. However, we were much impressed with the postoperative analgesic effect of a single high dose of pregabalin, especially in a major surgery like radical cystectomy.
A dose of 600 mg used once 2 h before surgery provided efficient analgesia and delayed first request of analgesia more than in other groups, with an overall reduction of total opioid consumption parallel to that of the 300 mg repeated after 12 h. The higher sedation, and incidence of dizziness with this single-high dose, was not extensive and required no intervention, and thus, we recommend this dose to be an integral part of the already-used postoperative analgesic regimens
9 Conclusion
Peri-operative pregabalin at doses of 300 mg and 600 mg reduced postoperative opioid consumption and prolonged time to first request of analgesia in patients underwent radical cystectomy, and a single preoperative dose of 600 mg is superior in analgesia to others, without serious side effects.
Conflict of interest
None of the authors has any conflict of interests.
Financial support
None of the authors has received financial support in order to accomplish this work.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- R.D.MillerAnesthesia2005Churchil LivingstonePhiladelphia
- H.KehletPostoperative opioid sparing to hasten recovery: what are the issues?Anesthesiology102200510831085
- S.KhademiF.GhaffarpasandH.R.HeiranM.J.YavariS.MotazedianM.DehghankhaliliIntravenous and peritonsillar infiltration of ketamine for postoperative pain after adenotonsillectomy: a randomized placebo controlled clinical trialMed Princ Pract202011433437
- G.A.McHughThe management of pain following day-case surgeryAnesthesiology572002270275
- D.R.GuayPregabalin in neuropathic pain: a more ‘pharma-ceutically elegant’ gabapentin?Am J Geriatr Pharmacother32005274287
- J.E.FramptonR.H.FosterPregabalin: in the treatment of pos-therpetic neuralgiaDrugs6520051118
- A.AgarwalS.GautamD.GuptaS.AgarwalP.K.SinghU.SinghEvaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomyBr J Anaesth1012008700704
- S.M.BurkeG.D.ShortenPerioperativepregabalin improves pain and functional outcomes 3 months after lumbar discectomyAnesth Analg110201011801185
- A.BuvanendranJ.S.KroinC.J.Della ValleM.KariM.MoricK.J.TumanPerioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trialAnesth Analg1102010199207
- O.MathiesenD.G.JørgensenK.L.HilstedW.TrolleP.StjernholmH.ChristiansenPregabalin and dexamethasone improves post-operative pain treatment after tonsillectomyActa Anaesthesiol Scand552011297305
- E.OzgencilS.YalcinH.TunaD.YorukogluY.KecikPerioperative administration of gabapentin 1200 mg day-1 and pregabalin 300 mg day-1 for pain following lumbar laminectomy and discectomy: a randomized, double-blinded, placebo-controlled studySingapore Med J522011883889
- P.SacerdoteM.BianchiL.GaspaniB.ManfrediA.MaucioneG.TernoThe effects of tramadol and morphine on immune responses and pain after surgery in cancer patientsAnesth Analg90200014111414
- C.P.TaylorThe biology and pharmacology of calcium channel alpha-2-delta proteinsCNS Drug Rev102004183188
- J.RosenstockM.TuchmanL.La MoreauxU.SharmaPregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trialPain11032004628638
- K.FinkD.J.DooleyW.P.MederN.Suman-ChauhanS.DuffyH.ClusmannInhibition of neuronal Ca (2+) influx bygabapentin and pregabalin in the human neocortexNeuropharmacology4222002229236
- C.WoolfM.ChongPreemptive analgesia-treating postoperative pain by preventing the establishment of central sensitizationAnaesth Analg771993362379
- B.D.LascellesA.E.WatermanP.J.CrippsA.LivingstonG.HendersonCentral sensitization as a result of surgical pain: investigation of the pre emptive value of pethidine for ovariohysterectomy in the ratPain621995201212
- D.J.RowbothamGabapentin: a new drug for postoperative pain?Brit J Anaesth962006152155
- A.TuranG.KayaB.KaramanliogluZ.PamukcuC.ApfelEffect of oral gabapentin on postoperative epidural analgesiaBrit J Anaesth962006242246
- M.J.PaechR.GayS.ChuaK.ScottT.ChristmasD.A.DohertyA randomized, placebo-controlled trial of preoperativepregabalin for postoperative pain relief after minor gynaecological surgeryAnesth Analg105200714491453
- R.JokelaJ.AhonenM.TaligrenM.HaanpaaK.KortillaA randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomyPain1342008106112
- R.JokelaJ.AhonenM.TaligrenM.HaanpaaK.KortillaPretreatment with pregabalin 75 or 150 mg with ibuprofen to control pain after day care gynaecological laproscopic surgeryBr J Anaesth1002008834840
- O.MathiesenL.S.JacobsenH.E.HolmS.RandallL.Adamiec-MalmstroemB.K.GraungaardPregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplastyBr J Anaesth1012008535541
- C.HillM.BalkenohlD.ThomasR.WalkerH.MatheG.MurrayPregabalin in patients with postoperative dental painEur J Pain52001119124
- W.IttichaikultholT.VirankabutraM.KunopartW.KhamhomP.PutarawuthichaiS.RungphetEffects of pregabalin on postoperative morphine consumption and pain after abdominal hysterectomy with/without salpingo-oophorectomy: a randomized double-blind trialJMed Assoc Thai92200913181323
- G.AkhavanakbariM.EntezariasK.IsazadehfarT.MirzarahimiThe effects of oral pregabalin on post-operative pain of lower limb orthopedic surgery: a double-blind, placebo-controlled trialPerspect Clin Res43201310.4103/2229-3485.115376
- E.M.TiippanaK.HamunenV.K.KontinenE.KalsoDo surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safetyAnesth Analg104200715451556
- C.MénigauxF.AdamB.GuignardD.I.SesslerM.ChauvinPreoperative gabapentin decreases anxiety and improves early functional recovery from knee surgeryAnesth Analg100200513941399
- A.GhaiM.GuptaS.HoodaD.SinglaR.WadheraA randomized controlled trial to compare pregabalin with gabapentin for postoperative pain in abdominal hysterectomySaudi J Anaesth532011252257