Abstract
Background
Anatomical variations in the thoracic nerves T7 to T11was found in up to 30% of the population as the anterior cutaneous branch of the nerves are formed before the rectus sheath and so do not penetrate the posterior wall of the rectus sheath. Posterior rectus sheath block was found to be effective for perioperative analgesia. We tested the efficacy of addition of anterior rectus sheath block to capture the anterior cutaneous branch of intercostal nerves as they emerge from the rectus muscle in anterior rectus sheath.
Method
Sixty-three ASA I/II adult patients listed for elective umbilical hernia repair were randomly allocated in one of three groups: Bupivacaine hydrochloride 0.25% was injected by ultrasound guided bilateral posterior rectus sheath in Group I (PRSB) and bilateral anterior and posterior rectus sheath in Group II (APRSB). Group III received bilateral anterior and posterior rectus sheath block using isotonic saline. Twenty-four hours postopetrative morphine consumption, Intraoperative rescue fentanyl dose, equivalent morphine dose in the recovery unit and first morphine dose were recorded. The quality of analgesia is assessed by Visual Analogue Scale for 24 h.
Results
Mean intraoperative rescue fentanyl dose was 19.23 ± 4.96 μg, 15.28 ± 2.75 μg and 12.85 ± 3.65 μg in control, PRSB and APRSB groups respectively (P < 0.001). The mean opioid consumptions in PACU was PRSB 3.47 ± 0.13 mg, APRSB 2.91 ± 0.15 mg and control 4.04 ± 0.56 mg respectively (P < 0.001). Significant difference in intraoperative rescue fentanyl was found between PRSB and APRSB group (P = 0.020). Also statistically significant difference was found between PRSB and APRSB groups in 24 h morphine consumption (P = 0.034).
Conclusion
Addition of ultrasound anterior rectus sheath block together with posterior rectus sheath block added more significant analgesia than if we perform posterior rectus sheath alone. This was evidenced by decrease in Intraoperative rescue fentanyl, PACU morphine analgesia, 24 h morphine and pain assessment score.
1 Introduction
1.1 Anatomical background
The key to understand abdominal wall nerve blocks is to know the applied anatomy of anterior abdominal wall and its innervation. From superficial to deep, there are the external oblique, internal oblique, and transverses abdominis. In addition, the paired rectus abdominis muscle forms a muscle layer either side of the midline (). The anterior abdominal wall can be described as the area surrounded by the inguinal ligament and the pelvic bone inferiorly, the costal margin and xiphoid process of the sternum superiorly, and laterally, the mid-axillary line [Citation1].
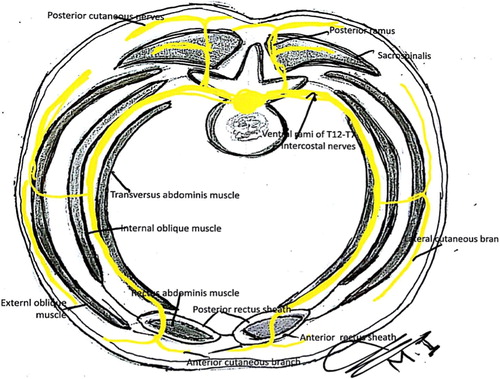
Between the internal oblique and transverses abdominis muscles lies a plane that corresponds with a similar plane in the intercostals spaces. In so doing they provide a compartment for the injection of local anesthetic. This plane contains the anterior rami of the lower six thoracic nerves (T7 to T12) and first lumbar nerve (L1), supplying the skin, muscles, and parietal peritoneum [Citation2].
At the costal margins, the thoracic nerves T7 to T11 enter this neurovascular plane of the abdominal wall, travelling along this plane to pierce the posterior wall of the rectus sheath as anterior cutaneous branches supplying the overlying skin [Citation2].
The nerves T7 to T9 emerge to supply the skin superior to the umbilicus. The T10 nerve supplies the umbilicus, whereas T11, the cutaneous branch of the subcostal T12, the iliohypogastric nerve, and the ilioinguinal nerve supply the skin inferior to the umbilicus [Citation3].
Rectus sheath block (RSB) has gained popularity for abdominal surgery in the era of fast track day case surgery [Citation4,Citation5]. Schleich firstly described it in 1899 [Citation6], aiming at deposition of local anesthetic (LA) in the virtual space between the posterior wall of the rectus abdominis muscle and its sheath. The anesthetic injected into this space is proposed to spread freely up and down and to block the terminal branches of the intercostal nerves before they leave the rectus sheath [Citation7].
The use of ultrasound (US) has helped to increase the feasibility and clinical applications for truncal block, allowing precise identification of the target structures and accurate visualization of the needle and LA spread [Citation8,Citation9]. US reopened the way for clinical application, study and refinement of RSB [Citation10–Citation15].
Anatomical variations was found in up to 30% of the population as the anterior cutaneous branch of the nerves are formed before the rectus sheath and so do not penetrate the posterior wall of the rectus sheath () [Citation16,Citation17].
Objectives
Strong evidence is lacking, and no studies to date have examined the addition of anterior rectus sheath block ARSB to posterior rectus sheath block PRSB will add more perioperative analgesia in umbilical herniorrhaphy.
We hypothesized that blocking the anterior coetaneous branch of intercostal nerves as they emerge from the rectus muscle in anterior rectus sheath will increase the chance and effectiveness of block.
For that we planned to test the hypothesis that adding US-guided ARSB to PRSB can offer more reduction in opioid consumption during the first 24 h after umbilical herniorrhaphy in comparison to US-guided PRSB and the systemic analgesia.
2 Methods
The study design was a prospective, randomized, observer double blinded trial with 3 parallel arms.
2.1 Inclusion and exclusion criteria
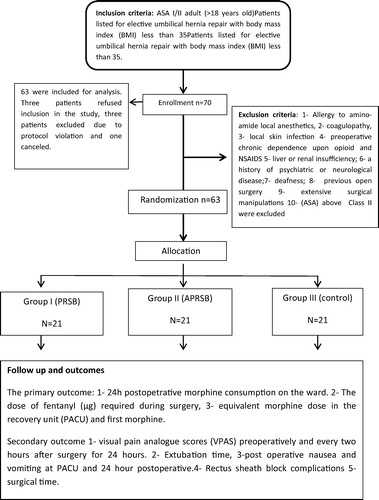
Inclusion criteria After approval from our Al Jedaani Hospital ethics committee, written informed consent was gained from 70 ASA I/II adult (>18 years old) at least 72 h before surgery by the surgical and anesthetic team. Patients listed for elective umbilical hernia repair with body mass index (BMI) less than 35.
Exclusion criteria included 1 - Patients allergic to amino-amide local anesthetics, 2 - presence of coagulopathy, 3 - local skin infection at the needle puncture sites, 4 - preoperative chronic dependence upon opioid and NSAIDS medications, 5 - liver or renal insufficiency; 6 - a history of psychiatric or neurological disease; 7 - deafness; 8 - previous open surgery; 9 - patients with that need of extending the incision and more extensive surgical manipulations than expected with more tissue trauma and loss of over 500 mL of blood during surgery e.g. hemicolectomy, 10 - American Society of Anesthesiologists (ASA) above Class II were excluded ().
All procedures were performed by the same surgeon. All patients received general anesthesia for the surgery. Preoperative investigations have been done according to local protocol designed to preoperative evaluation of patients undergoing surgical procedures.
Orientation about the use of visual analogue score (VAS) (10 cm marked line in which 0 cm referred to no pain and 10 cm to the worst pain)were done to all patients. Patients are asked to place a mark on the line to express the amount of pain that they are experiencing at a particular time. The distance between the end labeled “no pain” and the mark placed by the patient is measured in centimeters, to give a pain score between 0 and 10 cm. All patients received pre oxygenation with O2 100% for 5 min.
2.2 Study design
The study was conducted as randomized, double-blind, and placebo controlled trial. The surgeon, anesthetist and operating theater staff were blinded to the treatment group. Also, the patient and family, PACU, surgical ward nurses and anesthetist who collected the data were blinded.
2.3 Allocation and randomization
An independent person makes random allocation cards using computer-generated random numbers. He keeps the original random allocation sequences in an inaccessible third place and works with a copy. An independent nurse prepares syringes with “bupivacaine” and “saline” and puts them into envelopes according to the allocation orders. These syringes cannot be distinguished because they contain the same colored liquid with the same volume.
Sealed envelopes labeled with the following three groups: Group I (PRSB) for patients allocated for Bilateral Posterior Rectus Sheath Block; Group II (APRSB) for patients allocated for Bilateral Anterior and Posterior Rectus Sheath Block and Group III (Control) for patients allocated to receive Bilateral Anterior and Posterior Rectus Sheath Block using isotonic saline. Treatment allocation will be revealed by opening the envelope on the morning of surgery.
Group I (PRSB) received Bilateral Posterior Rectus Sheath Block using a dose of 10 mL of bupivacaine hydrochloride 0.25% using (Marcaine, Astra Zeneca UK) in each side. Deposition of 10 mL of isotonic saline in both anterior rectus sheath.
Group II (APRSB) received Bilateral Anterior and Posterior Rectus Sheath Block using bupivacaine hydrochloride 0.25% (total volume of 40 mL) deposited equally between the four (two anterior and two posterior) rectus sheath spaces.
Group III (Control) received Bilateral Anterior and Posterior Rectus Sheath Block using isotonic saline (total volume of 40 mL) deposited equally between the four (two anterior and two posterior) rectus sheath spaces. The skin incision was made 15 min after the block in the three groups.
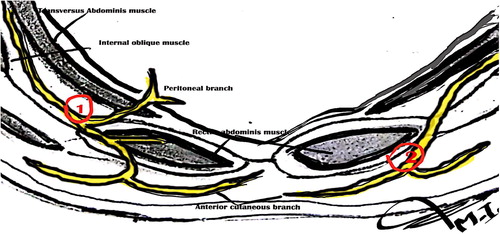
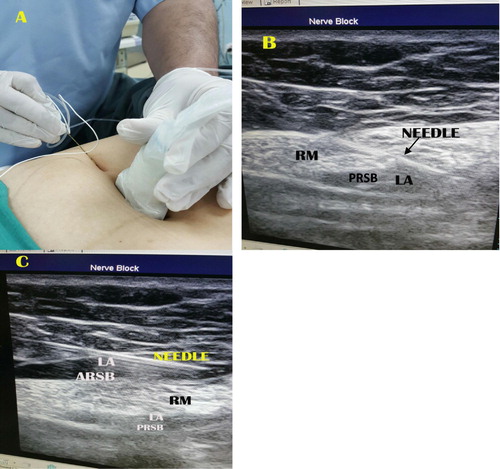
The skin was disinfected with povidine iodine 10% solution, and the transducer was covered with a sterile plastic cover and gel. The US probe was placed transversally to visualize rectus muscle at the T-10 level. The posterior rectus sheath and the fascia transversalis appear as twin of hyperechoic lines. An 85 mm, 20 G echoplex block needle (VYGON) connected to an injection line was introduced in plane with the US probe. The linear probe (7–13 MHz ultrasound transducer) in a lateral to medial direction at an angle of approximately 45° to the skin plane. No local anesthesia was performed ().
2.4 Technique
2.4.1 Posterior rectus sheath block
Under the guidance of ultrasound MacBook Pro-based ultrasound machines Terason t3200 portable ultrasound system, the needle was advanced gradually through the rectus muscle posteriorly toward its medial edge, approaching the rectus sheath. After negative aspiration, a bolus of 1 mL of 20 mL bupivacaine solution was injected. The solution was prepared by mixing 10 mL of bupivacaine 0.5% (Marcaine, Astra Zeneca UK) and 10 mL of saline solution, added with 0.1 mg epinephrine (final concentration bupivacaine 2.5 mg/mL, epinephrine 5 μg/mL). In case intramuscular injection of the LA occurred, advancing the needle until the rectus muscle was separated from the posterior rectus sheath by hydrodissection. At this point, the remaining anesthetic solution was deposited under US guidance (B). The same procedure was repeated on the opposite side.
2.4.2 Anterior rectus sheath block
Under the guidance of ultrasound, the needle was advanced gradually through the skin and subcutaneous tissues with the needle rest on the muscle and below the anterior rectus sheath (C). After negative aspiration, a bolus of 1 mL of 20 mL bupivacaine solution was injected. In case intramuscular injection of the LA occurred, withdrawal of the needle until the rectus muscle was separated from the anterior rectus sheath by hydrodissection. At this point, the remaining anesthetic solution was deposited under US guidance. All procedures were recorded as static and dynamic images.
2.5 Anesthesia
All patients received general anesthetic regimen, consisting of propofol (2.5 mg/kg), fentanyl (1 mcg/kg), and atracurium (0.5 mg/kg), A 0.2 mg/kg dose of additional acurium will be applied if needed Volatile agent used was Sevoflurane 1.5–2.0 minimum alveolar concentration in N2O/oxygen (fractional inspired oxygen of 0.4). Standart intraoperative monitoring included ECG and heart rate, pulse oximetry, automatic non-invasive blood pressure and end-tidal carbondioxide concentration. Fentanyl boluses were given in response to changes in hemodynamics (more than 15% increases in MAP and HR than the baseline values taken after induction by 5 min). If these parameters remain 15% above their baseline values, fentanyl boluses were repeated every 5 min. The total dose of fentanyl adminstered was documented.
Ventilator settings were adjusted to keep normocapnea of 35–40 mmHg and SPO2 between 94% and 100%. At the end of the procedure the neuromuscular block has been reversed with Neostigmine 50 μg/kg and Atropine 0.15 μg/kg. Extubation with patient awake and TOF response at 90% of control.
2.6 Surgical technique
Infra umbilical crescent incision for easy dissection of hernia sac through a smaller incision than the straight transverse incision. Then dissection and opening of hernial sac at its fundus to examine the viability of content before reduction. Examination and management of any adhesions between the abdominal content and abdominal wall before closure of umbilical defect.
Closure and reinforcement of umbilical defect by continuous suture and another row of proline suture invaginating the first suture. Fixation of suitable size of proline mesh using proline 3/0 suture. Insertion of portovac in the wound followed by closure of the wound without infiltration of bupivacaine for post-operative pain relief.
Postoperatively, patients were transferred to the post anesthesia care unit (PACU) for one hour. Recovery nurses, who were blinded to the group intervention. In the PACU, patients received intravenous analgesia of fentanyl 15–20 mcg IV or morphine 1–2 mg IV or pethidine 15–30 mg IV boluses. Administration of analgesia was decided if pain described as moderate or severe when asked about their pain intensity on a scale of mild, moderate, or severe. The criteria for discharge from the PACU were 1- absent or mild pain 2-absence of nausea and vomiting, 3-hemodynamic stability, and 4-alert or appropriately responsive to voice. On discharge, all patients had achieved a modified Aldrete score of ⩾9 [Citation20].
In the surgical ward, all patients received our hospital standard for postoperative analgesia regimen for such cases. It consists of paracetamol 1000 mg IV every 6 h, and in cases of moderate to severe pain, morphine 2–5 mg IV every 3 h as needed. Antiemetic medications including IV, dansetron 4 mg IV or metoclopramide 10 mg IV if needed.
Visual analogue score (VAS) is used to assess pain severity at 0 (recovery), 2, 4, 6, 12, and 24 postoperatively. Outcome data were collected by one of the team who was blind to the treatment groups.
The primary outcome was 24 h postopetrative morphine consumption on the ward which was calculated as the morphine dose equivalent to the opioid analgesia consumed (using opioid:morphine equivalents of 100 mcg i.v. fentanyl to 10 mg i.v. morphine; 75–100 mg IV pethidine to 10 mg i.v morphine [Citation21].
The dose of fentanyl (μg) required during surgery, equivalent morphine dose in the recovery unit (PACU) and first morphine dose also recorded.
Secondary outcome include the quality of analgesia as determined by comparing visual pain analogue scores (VPAS) preoperatively and every two hours after surgery for 24 h. Extubation time, post-operative nausea and vomiting at PACU and 24 h postoperative. Rectus sheath block complications (including local anesthetic systemic toxicity, vascular injury, intravascular injection of local anesthetic, local hematoma and visceral injury), surgical time (defined as the time between the incision and the completion of the dressing) were also documented ().
2.7 Statistical analysis
Statistical analysis was done using IBM-SPSS 20 software. The sample size of 21 per group was calculated assuming a 30% reduction in opioid use to provide 90% power at a significance level of 5% (P < 0.05 is considered significant). Allowing for a 20% drop-out rate, we planned to recruit a total of 70 subjects. The 30% assumed reduction was a conservative estimate based upon prior studies which describe the anatomical variation in which the anterior cutaneous branch of the nerves are formed before the rectus sheath and so do not penetrate the posterior wall of the rectus sheath [Citation18,Citation19].
Data are presented as median, range and interquartile range (IQR) or with mean and SD as appropriate. Morphine consumption did not follow a normal distribution and were compared with the Kruskall–Wallis test.
Categorical data were analyzed using the chi square (χ2) test. Normally distributed data was analyzed using a repeat-measures general linear model analysis of variance (ANOVA).
3 Results
Seventy patients were scheduled and data from 63 were included for analysis. Three patients refused inclusion in the study, three patients excluded due to protocol violation and one canceled. Patient characteristics were similar between groups ().
Table 1 Demographic data.
3.1 Opioid consumption
The control group required more intraoperative rescue fentanyl as compared to the PRSB and APRSB groups (). Mean fentanyl consumption (intraoperatively) was 19.23 ± 4.96 μg in the control group compared with 15.28 ± 2.75 μg μg and 12.85 ± 3.65 μg in both PRSB and APRSB groups respectively which show high significance statistically (P < 0.001).
Table 2 Comparison of analgesic efficacy of Anterior and posterior Rectus Sheath Block (APRSB) to both Posterior Rectus Sheath Block (PRSB) and control groups.
Median, Range and IQR for fentanyl consumption (intraoperatively) was 20 [10–26] 10 in the control groups compared with 15[10–20]3.5, and 10[10–20]5 in both PRSB and APRSB groups respectively.
The mean opioid consumptions in PACU () is (PRSB 3.47 ± 0.13 mg [95% CI, 3.11–3.56], APRSB 2.91 ± 0.15 mg [95% CI, 2.59–3.24] and control (4.04 ± 0.56 mg [95% CI, 3.78–4.30]; P < 0.001). Comparison between groups using independent t-test revealed significant differences between both PRSB and APRSB groups with control group (P < 0.001). Significant difference was found also between PRSB and APRSB groups (P = 0.020).
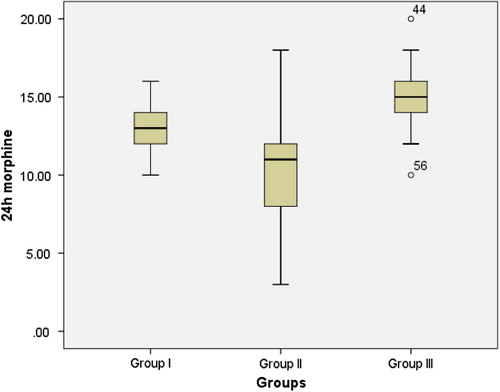
The mean 24 h morphine consumption () showed statistically high significant difference between groups (p value < 0.001 by using one way ANOVA). Significance confirmed by Kruskall–Wallis test due to abnormal distribution of data. Comparison between groups using independent t-test revealed significant differences between both PRSB and APRSB groups with control group (P = 0.002 and <0.001 respectively). Significant difference was found also between PRSB and APRSB groups (P = 0.034) ().
3.2 Pain assessment
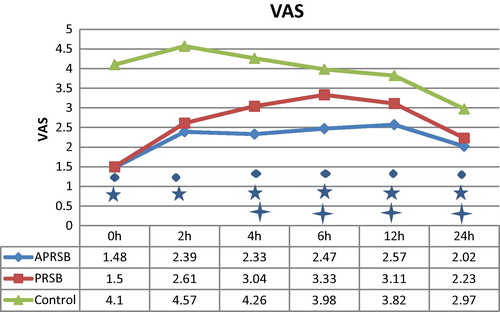
The mean VAS () of PRSB group became significantly higher than APRSB group after 4 h. The VAS was significantly lower in both APRSB and PRSB groups than in the control group at 0, 2, 4, 6, 12, and 24 h.
Duration of surgery (min) in ARSB, APRSB and control groups was 63.00 ± 7.67, 60.42 ± 7.04 and 63.61 ± 8.86 respectively (p = 0.38).
Time to extubation was significantly shorter in both ARSB and APRSB groups in comparison to control group (7.4 ± 2.2, 6.5 ± 2.9, and 9.7 ± 1.5 respectively p < 0.05). No side-effects related to APRSB and PRSB including PONV were observed 24 h after the block.
4 Discussion
We have attempted to examine the analgesic efficacy of adding US-guided anterior to posterior rectus sheath block (APRSB) in comparison with posterior rectus sheath block alone (PRSB) by 10 mL of bupivacaine 0.25% in each space bilaterally and placebo control group in adult patients undergoing umbilical hernia repair. Analgesic efficacy measured by intra and postoperative narcotic consumption and pain assessment using VAS for 24 h.
The study suggests that adding pre-incisional US-guided anterior to posterior rectus sheath block provide more analgesia than that obtained from posterior rectus sheath block alone which is proved to reduce PACU and 24 h morphine consumption in adult patients undergoing umbilical hernia repair when compared with placebo.
This assumption was based upon anatomical variations in the course of anterior cutaneous branch of intercostal nerves. Mori et al. [Citation18] and Yap et al. [Citation19] noted that all intercostal nerves from T-8 to T-11 pierce the rectus sheath at its lateral margin and run posterior to the rectus muscle for about 5 cm before entering the muscle (occasionally entering it at its lateral border).
In some cases the nerves enter directly into the lateral border of the muscle and may not lie in the target area. The most important point regarding rectus sheath blocks is that the nerves enter the lateral part of the sheath and blockade too medially may miss them. Both in plane and out of plane techniques have been described [Citation12,Citation22,Citation23].
The accurate course and distribution of the thoracolumbar nerves are lacking, and some studies describing these nerves has been contradictory. From a study by Courreges et al. [Citation17], it would seem that in up to 30% of the population the cutaneous branch of the intercostal nerves is formed before the rectus sheath and so does not pierce the posterior wall of the rectus sheath but instead runs anterior to the rectus muscle ().
Our study aimed at avoiding these variations by injecting in both anterior and posterior rectus sheath spaces to increase the chance of capturing the nerve.
The present study revealed that additional injection of LA at anterior rectus sheath together with posterior rectus sheath block added more significant analgesia than if we perform posterior rectus sheath alone.
We found significant changes in analgesic efficacy evidenced by decrease in Intraoperative rescue fentanyl, PACU morphine analgesia, 24 h morphine (), and VAS score () after the 4th hour of postoperative period when comparing the combined rectus sheath block group with only posterior rectus sheath group.
Our results showed high significance in reduction in postoperative opioid requirement, lower pain scores, and reduction in opioid-related side effects in both groups of rectus sheath block in comparison with control group. These results come in agreement with previous case reports [Citation24–Citation26] and retrospective studies [Citation27,Citation28] that concluded that RS block is a safe and effective technique of pain management.
Bashandy GMN et al. [Citation29] demonstrated that posterior rectus sheath block with general anesthesia provides more effective pain relief than general anesthesia alone. The VAS scores were better in patients of RSB group with less morphine utilization in the PACU. They also consumed less opioid in the early postoperative period with fewer side effects like respiratory depression, excessive sedation, and postoperative nausea and vomiting.
Gurnaney et al. [Citation12] demonstrated that ultrasound-guided PRSB provides more effective analgesia compared with local anesthesia infiltration (LAI) in the perioperative period in contrary to a previous study compared PRSB with LAI that found no difference in postoperative opioid use and pain scores [Citation30]. One of the differences between the two studies is the use of ultrasound guidance to perform the RSB in the first study.
Courreges et al. [Citation17] described a new technique to provide analgesia in 11 children undergoing the umbilical hernia repair: the para-umbilical block. They assumed that infiltration by LA in the middle of the rectus muscle, both above and below the anterior wall of the sheath would result in spread around the anterior cutaneous branches whatever the anatomical variation. They proved that the intraoperative analgesia, operative conditions and recovery were good in all patients.
Smith, et al. suggested that infiltration by LA in the middle of the rectus muscle, both above and below the anterior wall of the sheath, would result in spread around the anterior cutaneous branches whatever the anatomical variation [Citation31].
To capture these aberrant anterior cutaneous branches, Manassero et al. [Citation7], placed two injections of anesthetic bilaterally at the umbilicus level: One just under the anterior rectus sheath and one in the subcutaneous plane.de Jose Maria et al. [Citation11], in an attempt to minimize the effect of such anatomical variations, performed the block between the aponeurosis of the internal oblique and transversus muscles before the 10th intercostal nerves enter the rectus sheath.
Spread of the local anesthetic agent in the space between the anterior layer of the rectus sheath and the rectus muscles is restricted by the presence of the tendinous intcrscctions. There are no such intersections between the muscle and the posterior layer of the sheath [Citation7]. This point may be one limitation to our study but we think that this point didn’t affect our study results because the skin incision is at our injection level in almost all cases. Other thing is we thought if we did our block direction from medial to lateral but we did our best to evenly deposit local anesthetic in the space.
We concluded that addition of ultrasound anterior rectus sheath block together with posterior rectus sheath block added more significant analgesia than if we perform posterior rectus sheath alone. This was evidenced by decrease in Intraoperative rescue fentanyl, PACU morphine analgesia, 24 h morphine and pain assessment score.
Conflict of interest
The authors have no conflict of interest to declare.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- K.MooreA.DalleyClinically oriented anatomy7th ed.2014Lippincott Williams and WatkinsPhiladelphia (PA)
- J.G.McDonnellB.O’DonnellT.FarrellTransversus abdominis plane block: a cadaveric and radiological evaluationReg Anesth Pain Med322007399404
- Yarwood J, Berrill A. Nerve blocks of the anterior abdominal wall Continuing Education in Anaesthesia, Critical Care & Pain Advance Access published September 10, 2010.
- O.FinnertyJ.CarneyJ.G.McDonnellTrunk blocks for abdominal surgeryAnaesthesia65Suppl 120107683
- B.D.SitesR.BrullUltrasound guidance in peripheral regional an anesthesia: philosophy, evidence-based medicine, and techniquesCurr Opin Anaesthesiol1962006630639
- D.L.SchleichSchmerzlose operationen4th ed.1989Springer VerlagBerlin240258
- A.ManasseroM.BossolascoM.MeineriS.UguesC.LiarouL.BertolacciniSpread patterns and effectiveness for surgery after ultrasoundguided rectus sheath block in adult day-case patients scheduled for umbilical hernia repairJ Anaesthesiol Clin Pharm3132015349353
- M.S.AbrahamsJ.L.HornL.M.NolesM.F.AzizEvidence-based medicine: ultrasound guidance for truncal blocksReg Anesth Pain Med352010S36S42
- J.DolanP.LucieT.GearyM.SmithG.N.KennyThe rectus sheath block: accuracy of local anesthetic placement by trainee anesthesiologists using loss of resistance or ultrasound guidanceReg Anesth Pain Med342009247250
- H.WillschkeA.BösenbergP.MarhoferS.JohnstonS.C.KettnerO.WanzelUltrasonography-guided rectus sheath block in paediatric anaesthesia — a new approach to an old techniqueBr J Anaesth972006244249
- B.de Jose MariaV.GötzensM.MabrokUltrasound-guided umbilical nerve block in children: a brief description of a new approachPaediatr Anaesth1720074450
- H.G.GurnaneyL.G.MaxwellF.W.KraemerT.GoebelM.L.NanceA.GaneshProspective randomized observer-blinded study comparing the analgesic efficacy of ultrasound guided rectus sheath block and local anaesthetic infiltration for umbilical hernia repairBr J Anaesth1072011790795
- S.AzematiM.B.KhosraviAn assessment of the value of rectus sheath block for postlaparoscopic pain in gynecologic surgeryJ Minim Invasive Gynecol1220051215
- R.MalchowL.JaegerH.LamRectus sheath catheters for continuous analgesia after laparotomy without postoperative opioid usePain Med12201111241129
- P.CornishA.DeaconRectus sheath catheters for continuous analgesia after upper abdominal surgeryANZ J Surg77200784
- A.V.SkinnerG.R.lauderRectus sheath block: successful use in the chronic pain management of pediatric abdominal wall painPaediatr Anaesth172007 1203-1
- P.CourregesF.PoddevinD.LecoutrePara-umbilical block: a new concept for regional anaesthesia in childrenPaediatr Anaesth71997211214
- H.MoriK.AkitaY.HataAnatomical study of innervated transverse rectus abdominis musculocutaneous and deep inferior epigastric perforator flapsSurg Radiol Anat292007149154
- L.H.YapS.C.WhitenA.ForsterJ.H.StevensonThe anatomical and neurophysiological basis of the sensate free TRAM and DIEP flapsBr J Plast Surg5520023545
- J.A.AldreteModification to post anaesthesia score for use in ambulatory surgeryJ Perianaesth Nurs131998148155
- M.SasadaS.SmithDrugs in anaesthesia and intensive care3rd ed.2003Oxford University PressOxford
- P.HebbardY.FujiwaraY.ShibataC.RoyseUltrasound-guided transversus abdominis plane (TAP) blockAnaesth Intensive Care3542007616617
- P.HebbardSubcostal transversus abdominis plane block under ultrasound guidanceAnesth Anal10622008674675
- R.MalchowL.JaegerH.LamRectus sheath catheters for continuous analgesia after laparotomy – without postoperative opioid usePain Med12201111241129
- A.ShidoN.ImamachiK.DoiS.SakuraY.SaitoContinuous local anesthetic infusion through ultrasound-guided rectus sheath cathetersCan J Anaesth57201010461047
- A.H.AlsaeedA.ThallajN.KhalilN.AlmutaqA.AljazaeriUltrasound-guided rectus sheath block in children with umbilical hernia: case seriesSaud J Anaesth72013432435
- T.J.DuttonJ.S.McGrathM.O.DaughertyUse of rectus sheath catheters for pain relief in patients undergoing major pelvic urological surgeryBJU Int1132014246253
- A.R.GoddenM.J.MarshallA.S.GriceI.R.DanielsUltrasonography guided rectus sheath catheters versus epidural analgesia for open colorectal cancer surgery in a single centreAnn R Coll Surg Engl952013591594
- G.M.N.BashandyReducing postoperative opioid consumption by adding an ultrasound-guided rectus sheath block to multimodal analgesia for abdominal cancer surgery with midline incisionAnesth Pain Med432014e18263
- L.A.IsaacJ.McEwenJ.A.HayesM.W.CrawfordA pilot study of the rectus sheath block for pain control after umbilical hernia repairPaediatr Anaesth162006406409
- B.E.SmithM.SuchakD.SigginsJ.ChallandsRectus sheath block for diagnostic laparoscopyAnaesthesia431988947948