Abstract
Background
Co-administration of dexamethasone or neostigmine with local anesthetic solution for caudal block (CB) can prolong postoperative analgesia duration. We aimed to evaluate and compare the effectiveness of dexamethasone (0.1 mg/kg) versus neostigmine (2 μg/kg) when used as adjuvant to 0.25% bupivacaine for CB in children undergoing unilateral open inguinal hernia repair on the quality of postoperative analgesia.
Methods
105 children aged 1–6 years scheduled for unilateral open inguinal hernia repair were randomly allocated into three groups. Ultrasound guided CB was performed with 0.25% bupivacaine (0.75 ml/kg). 1 ml saline, dexamethasone (0.1 mg/kg) in 1 ml saline and neostigmine (2 μg/kg) in 1 ml saline were added in bupivacaine, bupivacaine-dexamethasone and bupivacaine-neostigmine respectively. Duration of postoperative analgesia, postoperative consumption of analgesic, the modified objective pain score, postoperative sedation and side effects were recorded.
Results
Duration of postoperative analgesia was prolonged in bupivacaine-dexamethasone and bupivacaine-neostigmine groups as compared to the bupivacaine group (P < 0.05). Bupivacaine-neostigmine provided the longest duration of postoperative analgesia. Postoperative analgesic consumption was lower in bupivacaine-dexamethasone and bupivacaine-neostigmine groups as compared to the bupivacaine group (P < 0.05). Bupivacaine-neostigmine provided lowest postoperative analgesic consumption. Postoperative nausea and vomiting was insignificantly different among the three groups.
Conclusion
Co-administration of dexamethasone (0.1 mg/kg) or neostigmine (2 μg/kg) with 0.25% bupivacaine for CB in pediatric patients undergoing unilateral open inguinal hernia repair prolonged postoperative analgesia duration and decreased postoperative analgesic utilization as compared to bupivacaine alone. Caudal bupivacaine-neostigmine provided more pronounced analgesic effect as compared to bupivacaine-dexamethasone.
1 Introduction
Caudal block (CB) is an oftentimes performed neuraxial technique to provide intraoperative and postoperative analgesia after infraumbilical surgery in pediatric. The most insistent disadvantage of single caudal injection is its short duration of analgesia [Citation1]. A handful of adjuvants such as fentanyl [Citation2], ketamine [Citation3], tramadol [Citation4] and midazolam [Citation3] can be added to the local anesthetics to improve the quality of the CB.
Neostigmine is a cholinesterase inhibitor that causes an increase of the acetylcholine concentration. Neostigmine has been frequently added to local anesthetics for caudal epidural analgesia [Citation3,Citation5].
Dexamethasone has running anti-inflammatory effects and has been tested for its analgesic efficacy. Epidural dexamethasone could trivialize the incidence and severity of postoperative pain in adults and children [Citation6,Citation7].
We evaluated and compared the effectiveness of dexamethasone (0.1 mg/kg) versus neostigmine (2 μg/kg) when used as adjuvant to 0.25% bupivacaine for CB in children undergoing unilateral open inguinal hernia repair on the quality of postoperative analgesia and their adverse effects.
2 Methods
After obtaining approval from the Tanta University Hospital Ethics Committee (30882/04/16), registration in the Pan African Clinical Trials Registry (PACTR201606001632982) and informed written consent from the patients' parents, this prospective double blinded randomized controlled clinical study was carried out on 105 child aged 1–6 years, of either gender, ASA I-II, undergoing elective open repair for unilateral inguinal hernia. The study was done in Tanta University Hospital, Pediatric surgery department, from April to September 2016. The duration of the study was 6 months. Proper explanation about the purpose of the study was given to every patient's parents. All patients' data were confidential with secret codes and in a private file for each patient. All given data were used for the current medical research only. Any unexpected risks may occur during the course of the research was discussed with the patients' parents and the ethical committee on time.
Patients with the following conditions were excluded from the study; infection at the injection site, bleeding diathesis, allergy to one of the study drugs, pre-existing neurological diseases, spinal diseases or known congenital anomaly of the spine.
The patients were randomized through a computer-generated randomization numbers in sealed opaque envelopes into three groups and parents of each patient chose the envelope which determined the group in which the patient was enrolled.
2.1 Group I (bupivacaine group)
After induction of general anesthesia, patients received ultrasound guided CB using 0.25% bupivacaine (0.75 ml/kg) with 1 ml saline.
2.2 Group II (bupivacaine – dexamethasone group)
After induction of general anesthesia, patients received ultrasound guided CB using 0.25% bupivacaine (0.75 ml/kg) with dexamethasone (Dexamethasone, Amriya Pharm Ind, Egypt. 4 mg/ml) 0.1 mg/kg diluted in normal saline to make 1 ml volume.
2.3 Group III (bupivacaine – neostigmine group)
After induction of general anesthesia, patients received ultrasound guided CB using 0.25% bupivacaine (0.75 ml/kg) with neostigmine (Epistigmin, Egyptian Int. Pharmaceutical Industries Co 0.5 mg/ml) 2 μg/kg diluted in normal saline to make 1 ml volume.
For all patients, the maximum volume used for CB would not be more than 20 ml and the total dose of bupivacaine would not exceed 2 mg/kg. Both dexamethasone and neostigmine were preservative free products. No premedication was given.
On arrival to operating room, routine monitoring including electrocardiography, non invasive arterial blood pressure and pulse oximetry were used. The mean arterial blood pressure (MAP) and heart rate (HR) were recorded before induction of general anesthesia (baseline).
Inhalational induction with 6–8% sevoflurane via face mask was done. A 22 G intravenous canula was inserted in the right hand after patient became unconscious. Endotracheal intubation was facilitated by atracurium 0.5 mg/kg. Patients were mechanically ventilated and ventilator parameters were set to keep endtidal CO2 between 30–35 mmHg. Anesthesia was maintained with sevoflurane (1.5–2%) in oxygen (50%) in air. The depth of anesthesia was adjusted to keep changes of hemodynamics; (MAP) and (HR); within the range of ±20% of the baseline.
Under complete aseptic conditions, ultrasound guided CB was performed in the lateral decubitus position. Ultrasound guided identification of the sacral cornua and hiatus was performed then a short beveled 22G needle was inserted midway from the sacral cornua and advanced into the caudal epidural space. After negative aspiration of blood or cerebrospinal fluid, the studied drugs were administered slowly and local anesthetic spread into the caudal epidural space was confirmed by detecting the turbulence in the caudal epidural space on the ultrasound image.
The studied drugs were prepared by an anesthesiology resident who had no subsequent role in the study. CB was performed by an anesthesiologist blinded to the utilized medications.
Surgical procedure was started after 15 min after performing the CB. During surgery, failure of caudal block was considered if the patient had an increased MAP or HR or both more than 20% above the baseline. In this case IV fentanyl 1 μg/kg was given and the patient was excluded from the study. Warm D5 1/2 normal saline was used at a rate of 5 ml/kg/h.
Intra-operative HR and MAP were recorded before induction of general anesthesia (T1), after induction but before caudal block (T2) and then at 10 min (T3), 20 min (T4), 30 min (T5) after caudal anesthesia and at the end of surgery (T6). Intra-operative hypotension was defined as a decrease of the blood pressure > 20% of the base line. Hypotension was treated by fluid infusion and ephedrine bolus (0.2 mg/kg) if needed. Intra-operative bradycardia was defined if the HR decreased more than 20% of the base line and treated by atropine 0.01 mg/kg.
At the end of surgery, children were extubated after reversal of muscle relaxant was done by neostigmine 0.05 mg/kg with atropine 0.02 mg/kg. Patients were transported to Post Anesthesia Care Unit (PACU) for 2 h. The recovery time (measured from discontinuation of sevoflurane to the time of spontaneous eye opening) was recorded. In the PACU, HR, respiratory rate, SpO2, systolic, diastolic and mean pressures were observed and any changes were recorded instantly.
The primary outcome was the duration of postoperative analgesia which was calculated from performing CB to the time for the 1st required postoperative dose of analgesic. The secondary outcomes included; total consumption of postoperative analgesia in the 1st 24 h and the incidence of side effects.
Postoperative pain was assessed using the ten points objective pain score [modified objective pain score (MOPS)] [Citation8] at arrival to PACU, 30 min, 1 h, 2 h, 4 h,6 h, 8 h,12 h, 16 h and 24 h postoperative. MOPS score consists of 5 items; crying, movements, agitation, posture and verbal; each item has score of 0–2 with total score ranges from 0 to 10 (). Postoperative analgesia, paracetamol 15 mg/kg, was infused if the MOPS score ≥ 4.
Table 1 Ten points objective pain score [Citation8].
The degree of motor blockade was evaluated immediately on admission to PACU, 30 min, 1 h, 2 h, 4 h, and 6 h by using Bromage scale [Citation9] (0; free movement of legs and feet, 1; free movement of feet with flexion of knee, 2; little movement of feet with no movement of knees, 3; no movements of feet or knees). Stimulation of feet and legs was performed for the evaluation of the motor block in young children who could not obey the verbal command.
Patients were evaluated for the degree of postoperative sedation at the same times for assessment of postoperative pain by using the following sedation score; (0: spontaneous eye opening, 1: eyes open in response to verbal stimulation, 2: eyes open in response to physical stimulation and 3: unarousable). Post-operative HR and MAP were recorded at the same times for the assessment of postoperative pain. Any complications i.e. nausea and vomiting, hypotension, bradycardia, respiratory depression and urine retention were recorded.
An anesthesiology resident blinded to the used medication for CB was responsible for performing all postoperative assessments.
Statistical analysis: Calculation of sample size relied upon the duration of postoperative analgesia after CB. Sample size was calculated utilizing statistical software STATA-9 (StataCorp LP, College Station, Texas, USA). Based on the results of the previous studies [Citation5,Citation10], a sample size of 31 children was required in each group to detect a difference at α level of 0.05 and study power of 80%. We used SPSS 16 (SPSS Inc., Chicago, IL, USA) for statistical analysis. The Kolmogorov-Smirnov test was performed to check the assumption of normality. Demographic data (age, weight, duration of surgery and hospital stay), duration of postoperative analgesia, MAP changes, HR changes and postoperative analgesic utilization were analyzed among the studied groups utilizing One-way ANOVA with post hoc Turkey's HSD Test. MOPS score, postoperative sedation score and Bromage scale were analyzed among the studied groups utilizing the Kruskal-Wallis test. Categorical data were described as patients' number or frequencies (%) and were compared utilizing the Chi-square test. P-value < 0.05 was considered significant.
3 Results
35 patients were enrolled in each group (); there was no significant statistical difference among the group's gender, age and weight ().
Table 2 Demographic data and perioperative criteria of the studied groups.
The duration of postoperative analgesia was prolonged in bupivacaine-dexamethasone (521.3 ± 101.1 min) and bupivacaine-neostigmine groups (792.7 ± 198.4 min) as compared to the bupivacaine group (295.9 ± 63.8 min); 95% confidence interval, (185.0–265.9) and (425.7–568.0) respectively (P < 0.05). Bupivacaine-neostigmine provided the longest duration of postoperative pain free period ().
Consumption of postoperative analgesia was significantly lesser in bupivacaine-dexamethasone and bupivacaine-neostigmine groups as compared to the bupivacaine group (P < 0.05). Bupivacaine-neostigmine group had the lowest postoperative analgesic consumption ().
On admission to PACU and up to 2 h postoperative, MOPS score was not statistically different among the three groups. At 4 h and 6 h postoperative, MOPS score was significantly lower in bupivacaine-dexamethasone and bupivacaine-neostigmine groups as compared to bupivacaine group (P < 0.05). At the same time, there was no significant difference between bupivacaine-dexamethasone group and bupivacaine-neostigmine group. At 8 h postoperative, MOPS score was significantly lower in bupivacaine-dexamethasone and bupivacaine-neostigmine groups compared to the bupivacaine group (P < 0.05). At the same time, MOPS score was significantly lower in bupivacaine-neostigmine group than bupivacaine-dexamethasone group (P < 0.05). At 12 h postoperative, MOPS score was significantly lower in bupivacaine-neostigmine group compared to bupivacaine-dexamethasone and bupivacaine groups (P < 0.05). At the same time, MOPS score was insignificantly different between bupivacaine-dexamethasone group and bupivacaine group (P > 0.05). At 16 h and 24 h postoperative, MOPS score was statistically comparable among the three studied groups (P > 0.05) ().
Table 3 Postoperative ten points objective pain score in the three groups.
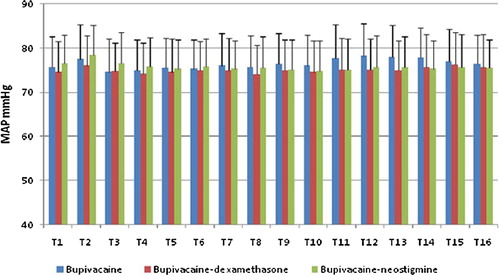
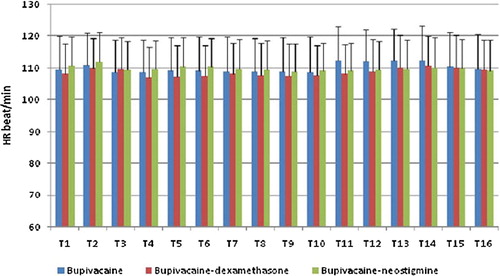
MAP and HR changes were comparable among the three studied groups at all times. and .
Postoperative sedation score and postoperative Bromage scale were not statically different among the three groups at all times of assessment (P > 0.05) ().
Table 4 Postoperative Bromage scale, sedation score and postoperative complications in the three groups.
5 patients, 3 patients and 7 patients developed postoperative nausea and vomiting in the bupivacaine group, bupivacaine-dexamethasone group and bupivacaine-neostigmine group respectively with statistically insignificant difference among the groups (P > 0.05) ().
Children were monitored for the possibility of occurrence of urine retention but no cases were detected.
4 Discussion
The present research showed that the co-administration of either dexamethasone 0.1 mg/kg or neostigmine 2 μg/kg to 0.25% bupivacaine for CB in children scheduled for unilateral open inguinal hernia repair lengthened postoperative pain free period and decreased postoperative analgesic consumption compared to the administration of bupivacaine alone without any significant adverse effects. The prolongation of postoperative analgesia and reduction of postoperative analgesic consumption were more marked with bupivacaine-neostigmine as compared to bupivacaine-dexamethasone.
The mechanism of analgesic effects of caudal dexamethasone is not fully cleared. Dexamethasone has direct action on the nerve membrane that causing local anesthetic effect which enhances the action of bupivacaine and prolongs its duration and this effect may explain the analgesic effect of caudal dexamethasone [Citation11]. Another possible mechanism is due to the action of dexamethasone on the spinal cord where dexamethasone may regulate nuclear factor-kB which is responsible for the development of pathological pain that can lead to inhibition of central sensitization and enhanced analgesic effect of CB [Citation12]. The nuclear factor-kB is expressed in the nervous system and has a chief responsibility for pathogenesis of pathological pain [Citation13].
The safety of epidural dexamethasone has been well documented [Citation14,Citation15].
As regards the analgesic effect of caudal dexamethasone administered in conjugation with local anesthetics solution, our results are on line with the results of Kim et al. [Citation6] who studied the effects of adding 0.1 mg/kg dexamethasone to ropivacaine 0.15% in 80 pediatric patients scheduled for ambulatory surgery, orchiopexy. Ropivacaine 0.15% (1.5 ml/kg) or ropivacaine 0.15% (1.5 ml/kg) with dexamethasone of 0.1 mg/kg were given to children for caudal epidural analgesia. They proved that co-administration of dexamethasone with ropivacaine significantly improved the analgesic quality. Choudhary et al. [Citation10] evaluated the analgesic effect of co-administration of dexamethasone 0.1 mg/kg to ropivacaine 0.2% for CB in 120 children underwent hernia repair. They concluded that the use of dexamethasone as an adjuvant to ropivacaine for CB produced longer post-operative pain relief period with lower pain score than those produced by ropivacaine. Several studies proved the analgesic effect of dexamethasone when added to local anesthetic solution [Citation7,Citation16].
The analgesic effect of caudal neostigmine is explained by inhibition of breakdown of the central neurotransmitter acetylcholine leading to increased acetylcholine concentration in cerebrospinal fluid [Citation17]. The analgesic effect is done via activation of spinal muscarinic M1 receptors and supraspinal muscarinic M1 and M2 and nicotinic cholinergic receptors [Citation18,Citation19].
As regards the analgesic effect of neostigmine 2 μg/kg when added to bupivacaine, our results are in consistence with the results of Kumar et al. [Citation3] who studied the effects of adding ketamine (0.5 mg/kg), midazolam (0.05 mg/kg), and neostigmine (2 μg/kg) to 0.25% bupivacaine for CB in 80 pediatric patients underwent unilateral inguinal herniotomy. They concluded that co-administration of neostigmine or midazolam with bupivacaine for CB associated with prolonged postoperative analgesia.
Karaaslan et al. [Citation5] studied the effects of adding 2 or 4 μg/kg neostigmine to levobupivacaine 0.25% (1 ml/kg) for CB in 60 pediatric patients scheduled for genito-urinary surgery. They concluded that co-administration of neostigmine with levobupivacaine lengthened the postoperative pain free period without significant adverse events and 2 μg/kg neostigmine seemed to be the optimal dose. Mahajan et al. [Citation20] compared the effects of adding three doses of neostigmine (2, 3 or 4 μg/kg) to 0.25% bupivacaine (0.5 ml/kg) for CB in 80 children underwent surgery for hypospadias repair. They proved that the administration of neostigmine in doses of 2, 3 or 4 μg/kg with bupivacaine for CB resulted in better analgesic efficacy (approximately 16–17 h) with reduction of postoperative analgesic requirement without significant side effects as compared to bupivacaine alone. The analgesic effect of caudal neostigmine when added to local anesthetic solution was documented in previous studies [Citation4,Citation21].
On the other hand, Memiş et al. [Citation22] concluded that administration of neostigmine (1 μg/kg) with bupivacaine 0.25% for CB did not improve postoperative analgesic duration in comparison to bupivacaine alone. The difference between their results and other investigators who documented the analgesic effectiveness of caudal neostigmine may be contributed to the low dose of neostigmine used by Memis et al. Bhardwaj et al. [Citation23] studied the effects of adding three doses of neostigmine (2, 3 or 4 μg/kg) to bupivacaine (0.75 ml/kg) for CB in 120 children underwent urethroplasty surgery. They demonstrated that the co-administration of neostigmine with bupivacaine for CB did not lengthen the postoperative analgesia duration.
Animals and humans studies documented the safe utilization of neostigmine administrated either epidural or intrathecal [Citation24,Citation25].
As regards the postoperative nausea and vomiting; it was insignificantly increased in bupivacaine-neostigmine group (20% of patients) as compared to bupivacaine group (14.3% of patients) and bupivacaine-dexamethasone group (8.6% of patients). These results as regards the incidence of postoperative nausea and vomiting with caudal bupivacaine- neostigmine are in consistent with the results of previous studies [Citation3,Citation5,Citation21]. On the other hand, other studies documented high incidence of postoperative nausea and vomiting up to 30% after caudal administration of neostigmine [Citation26,Citation27].
5 Conclusion
Co-administration of dexamethasone (0.1 mg/kg) or neostigmine (2 μg/kg) with 0.25% bupivacaine for CB in pediatric patients undergoing unilateral open inguinal hernia repair prolonged postoperative analgesia duration and decreased postoperative analgesic utilization as compared to bupivacaine alone. Caudal neostigmine provided more pronounced analgesic effects as compared to dexamethasone.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- P.SilvaniA.CamporesiM.R.AgostinoI.SalvoCaudal anesthesia in pediatrics: an updateMiner Anestesiol722006453459
- Y.KawaraguchiT.OtomoC.OtaN.UchidaA.TaniguchiS.InoueA prospective, double-blind, randomized trial of caudal block using ropivacaine 0.2% with or without fentanyl 1 μg/kg in childrenBr J Anesth972006858861
- P.KumarA.RudraA.K.PanA.AcharyaCaudal additives in pediatrics: a comparison among midazolam, ketamine, and neostigmine coadministered with bupivacaineAnesth Analg10120056973
- R.TaheriS.ShayeghiS.S.RazaviEfficacy of bupivacaine-neostigmine and bupivacaine-tramadol in caudal block in pediatric inguinal herniorrhaphyPediatr Anesth202010866872
- K.KaraaslanN.GulcuH.OzturkA.SarpkayaC.ColakH.KocogluTwo different doses of caudal neostigmine co-administered with levobupivacaine produces analgesia in childrenPediatr Anesth1952009487493
- E.M.KimJ.R.LeeB.N.KooY.J.ImH.J.OhJ.H.LeeAnalgesic efficacy of caudal dexamethasone combined with ropivacaine in children undergoing orchiopexyBr J Anesth1122014885891
- S.ThomasS.BeeviEpidural dexamethasone reduces postoperative pain and analgesic requirementsCan J Anesth532006899905
- G.A.M.WilsonE.DoyleValidation of three pediatric pain scores for use by parentsAnesthesia51199610051007
- P.R.BromageA comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesiaActa Anesth Scand919655569
- S.ChoudharyN.DograJ.DograP.JainS.K.OlaB.RatreEvaluation of caudal dexamethasone with ropivacaine for postoperative analgesia in pediatric herniotomies: a randomised controlled studyIndian J Anesth6020163033
- A.JohanssonJ.HaoB.SjolundLocal corticosteroid application blocks transmission in normal nociceptive C-fibresActa Anesthesiol Scand341990335338
- K.De BosscherW.Vanden BergheG.HaegemanThe interplay between the glucocorticoid receptor and nuclear factor-kappa B or activator protein-1: molecular mechanisms for gene repressionEndocr Rev242003488522
- E.NiederbergerG.GeisslingerThe IKK-NF-kappa B pathway: a source for novel molecular drug targets in pain therapy?FASEB J22200834323442
- F.M.AhadianK.McGreevyG.SchulteisLumbar transforaminal epidural dexamethasone: a prospective, randomized, double-blind, dose–response trialReg Anesth Pain Med362011572578
- R.MaX.WangC.LuDexamethasone attenuated bupivacaine induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanismNeuroscience1672010329342
- G.T.YousefT.H.IbrahimA.KhderM.IbrahimEnhancement of ropivacaine caudal analgesia using dexamethasone or magnesium in children undergoing inguinal hernia repairAnesth Essays Res820141319
- T.L.YakshR.DirksenG.J.HartyAntinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and catEur J Pharmacol11719858188
- M.NaguibT.L.YakshAntinociceptive effects of spinal cholinesterase inhibition and isobolographic analysis of the interaction with μ and α2 receptor systemsAnesthesiology80199413381348
- H.BouazizC.TongJ.C.EisenachPostoperative analgesia from intrathecal neostigmine in sheepAnesth Analg80199511401144
- R.MahajanV.K.GroverP.ChariCaudal neostigmine with bupivacaine produces a dose-independent analgesic effect in childrenCan J Anesth512004702706
- A.TuranD.MemişU.N.BaşaranB.KaramanlioğluN.SütCaudal ropivacaine and neostigmine in pediatric surgeryAnesthesiology982003719722
- D.MemişA.TuranB.KaramanlioğluG.KayaN.SütZ.PamukçuCaudal neostigmine for postoperative analgesia in pediatric surgeryPediatr Anesth32003324328
- N.BhardwajS.YaddanapudiB.GhaiJ.WigNeostigmine does not prolong the duration of analgesia produced by caudal bupivacaine in children undergoing urethroplastyJ Postgrad Med532007161165
- T.L.YakshM.R.GrafeS.MalkmusM.L.RathbunJ.C.EisenachStudies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogsAnesthesiology821995412427
- F.RoelantsM.RizzoP.Lavand’hommeThe effect of epidural neostigmine combined with ropivacaine and sufentanil on neuraxial analgesia during laborAnesth Analg96200311611166
- Y.K.BatraV.K.AryaR.MahajanP.ChariDose response study of caudal neostigmine for postoperative analgesia in pediatric patients undergoing genitourinary surgeryPediatr Anesth132003515521
- N.AlmenraderM.PassarielloG.D'AmicoR.HaibergerP.PietropaoliCaudal additives for post-operative pain management in children: S (+) ketamine and neostigminePediatr Anesth152005143147