Abstract
Background
This study evaluated impact of intraoperative goal-directed therapy (GDT) judged by changes of stroke volume variation (SVV) and cardiac index (CI) using the Vigileo/FloTrac system on postoperative (PO) morbidities and mortality rates in high risk patients scheduled for major abdominal surgeries in comparison to conventional fluid therapy (CT).
Methods
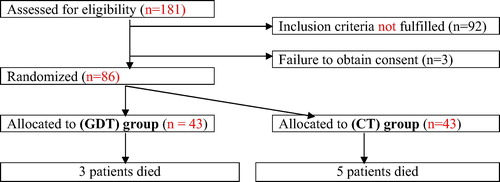
86 patients were randomly allocated into one of two equal groups: CT group = 43 patients received crystalloid solution and on demand bolus of 250 ml colloids with possible addition of vasopressor or inotrope to target MAP at 60–90 mmHg, CVP at 8–12 mmHg and urine output at >0.5 ml/kg/hr and GDT group = 43 patients received crystalloid fluid therapy (FT) and colloids (3 ml/kg) with possible addition of vasopressor or inotrope according to predefined protocol with a target CI 2.5 L/min/m2, SVV < 12% with MAP > 65 mmHg. Physiological and Operative Severity Score for Enumeration of Mortality and Morbidity (POSSUM) was used to predict morbidity and mortality rates. Study outcomes included ICU and total hospital morbidity and mortality rates and length of stay (LOS).
Results
Intraoperative GDT reduced ICU morbidity rate (16.3%) than the POSSUM predicted rate (39.78%) and significantly (p = 0.039) than CT group (37.2%), while in CT group ICU morbidity rate coincided with POSSUM predicted rate (42.86%). ICU and total hospital LOS were significantly shorter with GDT group than with CT group. However, mortality rates weren’t significantly lower with GDT group than CT group (7% vs. 11.6%). The applied protocol for intraoperative GDT reduced significantly crystalloid infusion and despite of significantly higher amount of received colloids, the total amount of FT was significantly less than CT group.
Conclusion
The applied protocol for intraoperative GDT provided significant reduction of PO morbidities, ICU and hospital LOS but couldn‘t significantly reduce mortality rates in high risk patients scheduled for major abdominal surgeries.
1 Introduction
Perioperative hemodynamic optimization improves postoperative (PO) outcome for patients undergoing high-risk surgery [Citation1]. Conventional liberal, restricted and goal-directed fluid management have different influences on PO complications and mortality [Citation2]. Targets of goal-directed hemodynamic management are to optimize stroke volume (SV) by fluid therapy; maintain target mean arterial pressure (MAP) by vasopressor therapy and cardiac index by inotropic therapy [Citation3].
Dynamic variation parameters including systolic pressure variation, pulse pressure variation and SV variation (SVV) measured using the Pulse Index Continuous cardiac system can both track changes in blood volume and to predict fluid responsiveness [Citation4]. Pleth Variability Index derived from the finger pulse oximeter waveform could predict fluid responsiveness during surgery in ventilated patients [Citation5].
Cardiac SV estimated by ultrasound Doppler and by arterial blood pressure curve showed parallel variations beat-to-beat in cardiac SV, whereas impedance cardiography did not appear to track beat-to-beat changes [Citation6]. The Vigileo/FloTrac system (Edwards Lifescience, Irvine, CA, USA), is a SVV, cardiac output (CO) and cardiac index (CI) monitoring device, based on arterial pulse contour; therefore, it offers the possibility of the almost beat-to-beat measurements of CO, CI and SVV [Citation7]. Multiple studies assured the accuracy of Vigileo/FloTrac device in assessing SVV, CO and CI in numerous settings [Citation8,Citation9].
The primary outcome of the current study was to assess the impact of intraoperative goal-directed therapy (GDT) as judged by changes of SVV and CI using the Vigileo-FloTrac system on the frequency of PO complications encountered during ICU stay, ICU and hospital length of stay (LOS) in high risk patients scheduled for major abdominal surgeries. Secondary outcome included ICU mortality, the frequency of ICU re-admission and total hospital morbidity and mortality.
2 Methods
The current prospective comparative study was conducted at Ain-Shams University hospitals, since June 2014 till May 2016. The study protocol was approved by the Local Ethical Committee and enrolled patients, fulfilling the inclusion criteria, signed written fully informed consent prior to inclusion. Inclusion criteria included high-risk patients scheduled for major abdominal surgeries with anticipated operative time of >120 min or blood loss of >20% of blood volume.
High-risk was defined if patient was of ASA grade II or III [Citation10] and had at least one of the six independent predictors of complications included in Lee Revised Cardiac Risk Index: high-risk type of surgery, history of ischemic heart disease, congestive heart failure, cerebrovascular disease, or preoperative treatment with insulin, and/or preoperative serum creatinine >2.0 mg/dl. Each risk factor was assigned one point and major cardiac complications are rated as Class I-IV for points 0, 1, 2, or ≥3 [Citation11].
Patients were randomly allocated, using sealed envelopes prepared by blinded assistant and chosen by patient him/herself, into two equal groups (n = 43 patients each) according to modality of intraoperative monitoring and hemodynamic therapy. All patients were premedicated by intravenous (IV) injection of metoclopramide 10 mg, ranitidine 50 mg and midazolam 0.01 mg/kg. All patients received preoperative IV infusion of Lactated Ringer (LR) solution at rate of 2 ml/kg/hr at 8.00 AM of the day of surgery till admission to operating room.
Before induction of anesthesia, central venous catheter was inserted via the right internal jugular vein for measurement of central venous pressure (CVP) and fluid administration. Antero-posterior chest X-ray was performed for assurance of proper catheter insertion. An arterial line (20G, BD arterial cannula, BD critical care system Ltd., Singapore) was inserted into radial artery of non-dominant forearm and baseline invasive arterial blood pressure (ABP) measurement was determined and blood samples were obtained for baseline laboratory estimations. Standard monitoring for all patients included invasive monitoring for arterial blood pressure, CVP, and urine output (UOP) and non-invasive monitoring of heart rate (HR) using 5-lead ECG, SpO2 and ETco2.
General anesthesia was induced for both groups with IV injection of fentanyl 1–fentanyl 1-2 μg/kg2 μg/kg, thiopental 3–thiopental 3-5 mg/kg5 mg/kg (as titratable dose), atracurium 0.5 mg/kg. Patients were maintained on controlled mask ventilation with 100% oxygen until adequately relaxed and then endotracheal tube was inserted within 3–5 min. After intubation, mechanical ventilation was initiated with 100% oxygen using volume controlled mode, tidal volume of 6–8 ml/kg and PEEP 3–5 cmH2O with the respiratory rate was set to maintain normocapnia and the peak pressure of the ventilator was limited to 40 cmH2O. Incremental doses of fentanyl (0.5–1.0 μg/kg) and atracurium (0.1 mg/kg) were given according to needs in addition to isoflurane 1–2% as an inhalational anesthetic.
2.1 Intraoperative (IO) monitoring and fluid therapy (FT)
2.1.1 Conventional fluid therapy (CT) group
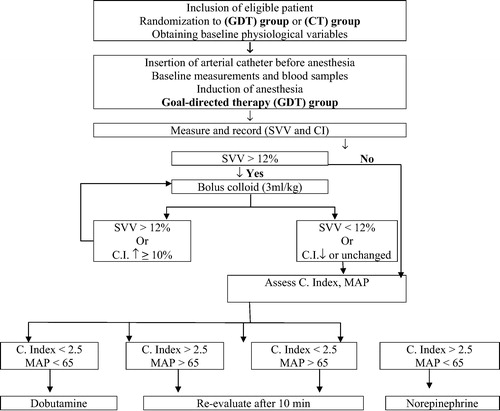
| • | The endpoint for patients of CT group was to keep MAP ranging between 60 and 90 mmHg with CVP in range of 8–12 mmHg and UOP of >0.5 ml/kg/h. | ||||
| • | Maintenance IV infusion FT was provided in the form of crystalloid solution (LR solution) so as to maintain MAP in range of 60–90 mmHg with CVP in range of 8–12 mmHg and UOP at rate of >0.5 ml/kg/h. However, according to hemodynamic monitoring; if MAP < 65 mmHg or UOP < 0.5 ml/kg/h and CVP < 8 mmHg, more than 20 min, a bolus of 250 ml of 6% hydroxyethyl starch in saline (6% HES 130/0.4; Voluven; Fresenius Kabi AG, Bad Homburg, Germany) was given; but if CVP was 8–12 mmHg with the same former drop of MAP and UOP; nor-epinephrine infusion at rate of 50 ng/kg/min was started in addition to a similar bolus of colloid FT. In case of CVP > 12 mmHg with MAP < 65 mmHg, dobutamine infusion at initial rate of 5 μg/kg/min was started. | ||||
2.1.2 Goal-directed therapy (GDT) group
| • | Patients received enhanced hemodynamic monitoring with the Vigileo/FloTrac device (Edwards Lifesciences, Irvine, CA, USA). The arterial line was connected to the Vigileo monitor (software version 1.14) via the FloTrac pressure transducer and all intravascular pressure measurements are referenced to mid-axillary line level. Both CI and SVV were continuously monitored and recorded every 20-min.
| ||||||||||
2.1.3 Both groups
| • | Patients of both groups were continuously monitored for HR, ABP with MAP, CVP and UOP were recorded hourly. | ||||
| • | Blood loss was initially substituted with fluids according to the protocol applied for each group, but if hemoglobin concentration reached <9 g/dl, transfusion of packed red blood cells (PRC) and fresh frozen plasma (FFP) was started. | ||||
| • | Amount of IO blood loss, fluids and blood products intake, need of patients for inotropes and vasopressors were reported. | ||||
| • | Venous blood samples were obtained before induction of anesthesia and at time of ICU admission and collected in sodium fluoride containing tubes for estimation of blood lactate level [Citation12]. | ||||
2.2 ICU monitoring
| • | All patients were admitted to ICU and received maintenance FT as crystalloid fluid at rate of 1–1.5 ml/kg/h. | ||||
| • | HR, MAP, CVP, UOP and blood lactate level were measured on ICU admission and at 6, 12, 24, and 48-h postoperative (PO) in both groups. | ||||
| • | Patients were evaluated using the Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (POSSUM) [Citation13]. | ||||
| • | Perioperative morbidities and mortality were assessed daily throughout ICU stay by an assistant who was blinded about patients' grouping and intraoperative management. After discharge from ICU, patients were assessed daily by a surgeon blinded about patients' grouping and intraoperative management. | ||||
2.3 Statistical analysis
Sample size was calculated using the standard nomogram proposed by Kraemer & Thiemann [Citation14] and a sample size of >40 patients was determined to be sufficient to detect a difference at the 5% significance level and give the trial 80% power [Citation15]. Sample size and power were re-calculated and assured using Power and Sample Size Calculation Software program provided by Department of Biostatistics, Vanderbilt University. Obtained data were presented as mean with standard deviation, numbers and percentages. Results were analyzed using One-way ANOVA with post hoc Tukey HSD Test for parametric data and Chi-square test (X2 test) for non-parametric analysis of numbers and ratios and t-test for intergroup and intra-group comparisons. Statistical analysis was conducted using the IBM SPSS (Version 23, 2015) statistical package. P value <0.05 was considered statistically significant.
3 Results
Flow chart for patients of this study is shown in ().
This study included 86 patients with mean age of 57.5 ± 7.6; range: 38–69 years. All patients were high-risk, scheduled for major abdominal surgeries with non-significant (p > 0.05) difference between both groups (). All surgeries were conducted uneventfully without intraoperative mortalities or morbidities with non-significant difference between both groups as regards time-lapse till conduction of surgery, operative time, amount of blood loss and frequency of the need for RBC and FFP transfusion, nor-epinephrine or dobutamine infusions (). Intraoperatively, patients of CT group received significantly higher amount of crystalloid FT, while patients of GDT received significantly higher amount of colloid FT. Intraoperative hemodynamic parameters showed non-significant (p > 0.05) difference between both groups. Similarly, UOP as a guide for tissue perfusion showed non-significant (p > 0.05) difference between both groups ().
Table 1 Demographic data of patients of both groups.
Table 2 Intraoperative data of patients of both groups.
Table 3 Intraoperative hemodynamic and UOP data of patients of both groups.
During 1st 24-h of ICU stay, patients of CT group received significantly (p = 0.001) larger amount of crystalloid FT, but non-significantly (p > 0.05) smaller amount of colloid FT. Therefore, CT patients received collectively a significantly (p = 0.001) higher total amount of FT than patients of GDT group. The frequency of patients required PRC transfusion, nor-epinephrine infusion or dobutamine infusion showed non-significant (p > 0.05) difference between both groups ().
Table 4 ICU fluid therapy and medication received by patients of both group during the 1st 24-h ICU stay.
Postoperative blood lactate levels were significantly higher in comparison to preoperative levels in both groups reaching a maximum at time of admission to ICU and then declined progressively till 24-h PO when the difference was non-significant versus preoperative levels. On the other side, estimated blood lactate levels were significantly higher in patients of CT group compared to patients of GDT group throughout the 1st 24-h PO ().
Table 5 Mean blood lactate levels estimated throughout 1st 24-h ICU stay compared to preoperative levels:
At admission to ICU, calculated morbidity and mortality according to POSSUM score showed non-significant (p > 0.05) difference between patients of both groups. Number of patients developed PO events during ICU stay was significantly (p = 0.039) higher among patients of CT group compared to patients of GDT group. The actual total morbidity rate reported in CT group was coincident with POSSUM predicted morbidity rate, while was lower in case of GDT group. During ward stay; 14 patients from both groups developed another morbidity and 4 patients (3 in CT and 1 in GDT group) required ICU re-admission with non-significantly (p > 0.05) higher frequency among patients of CT group. Mean duration of ICU stay was significantly (p = 0.001) shorter, while total hospital length of stay was non-significantly (p > 0.05) shorter for patients of GDT group than those of CT group. Only three patients; 2 in CT and one in GDT groups died during ICU stay and another 5 patients; 3 in control and 2 in GDT group died during ward stay with non-significant difference in favor of GDT group. Total morbidity rate was significantly (p = 0.028) lower, while total mortality rate was non-significantly (p > 0.05) lower among patients of GDT group compared to those of CT group ().
Table 6 Outcome of patients of both groups.
4 Discussion
The primary target of the current study was the ICU and total hospital morbidity and mortality rates; intraoperative GDT using minimally invasive monitoring of cardiac index (CI) and stroke volume variations (SVV), allowed significant reduction of ICU morbidity rate than conventional FT (CT) and than expected by POSSUM scoring. However, the reported ICU morbidity rate after CT was coincident with POSSUM predicted morbidity rate. These findings spotlight on the possibility of reducing postoperative (PO) morbidities in high-risk patients undergoing major surgeries through hemodynamic optimization depending on direct cardiac function monitoring more than reliance on conventional monitoring using central venous pressure (CVP) and arterial blood pressure measurements.
The impact of intraoperative GDT on postoperative morbidity is still a matter of discrepancy, where Lin et al. [Citation16] and Lahtinen et al. [Citation17] reported non-significant difference in total complication rates between goal-directed and conventional fluid therapy patients' groups. On the other hand, Manecke et al. [Citation18] found GDT decreased morbidity rate with subsequent gross costs saving and Muñoz et al. [Citation19] found implementation of GDT significantly reduced PO nausea and vomiting after laparoscopic sleeve gastrectomy in morbidly obese patients. Also, Bacchin et al. [Citation20] reported that patients received GDT had fewer pulmonary complications and faster return of bowel function after major spine surgery. Furthermore, Kratz et al. [Citation21] reported significantly fewer severe complications and renal failure after implementation of GDT for patients underwent pancreatic resection.
Moreover, multiple recent meta-analyses of published prospective randomized controlled trials comparing GDT with standard care found application of GDT based on dynamic parameters significantly reduced PO morbidity [Citation22], was associated with significant reduction in ICU and hospital length of stay [Citation23] and significantly lower frequency of abdominal complications, wound infection and PO hypotension [Citation24].
Venous not arterial blood lactate levels were estimated depending on that documented by Kruse et al. [Citation25] who found that peripheral venous lactate levels are highly correlated with arterial blood lactate levels, thus establishing that either method can be utilized. PO blood lactate levels were significantly higher in patients of both groups compared to their preoperative levels, but were significantly lower in patients of GDT than those of CT group; these findings spotlight on proper tissue perfusion and oxygenation with maintenance of metabolism towards aerobic arm, facilitating lactate consumption through Cori cycle for glucose synthesis and metabolism. In line with these findings; Bacchin et al. [Citation20], Benes et al. [Citation26] and Yu et al. [Citation27] found lactate levels at the end of surgery were lower with GDT compared to CT.
The current study also reported significantly shorter ICU and total duration of hospital stay for patients received intraoperative GDT compared to CT. Review of literature showed discrepant opinion about the impact of GDT on ICU and hospital stay of patients underwent major non-cardiac surgeries; wherein Lin et al. [Citation16], Lai et al. [Citation28] Srinivasa et al. [Citation29,Citation30] reported no difference in hospital LOS between patients undergoing major elective surgery under CT or GDT, while Kratz et al. [Citation21] and Som et al. [Citation24] reported that GDT non-significantly reduced ICU and hospital stay. On the other hand, Manecke et al. [Citation18], Benes et al. [Citation22], Joosten et al. [Citation31] found GDT management reduced ICU and hospital stay. Recently, Lahtinen et al. [Citation17] and Muñoz et al. [Citation19], Bacchin et al. [Citation20], Rollins & Lobo [Citation23] and Yu et al. [Citation27] reported a shorter ICU stay for patients received GDT than patients received CT during major surgeries.
However, the reported mortality rate was non-significantly lower with GDT than CT (7% vs. 11.6%). In line with this finding, Rollins & Lobo [Citation23] and Som et al. [Citation24] found the use of GDT in major surgical patients does not decrease PO hospital 30-day mortality rate versus CT, while Kratz et al. [Citation21] reported non-significant difference in mortality rate of patients underwent pancreatic surgery before and after implementation of GDT.
The applied protocol for GDT significantly reduced amount of intraoperative crystalloid infusion and despite of the significantly higher amount of received colloid, the total amount of FT was significantly less than with CT. On contrary, Lin et al. [Citation16] and Srinivasa et al. [Citation29,Citation30] reported no significant difference in the overall fluid volumes administered intraoperatively between patients with and without GDT, but patients in GDT group received significantly higher volume of colloid fluids. However, in hand with the current study, multiple recent studies reported similar significant reduction of administered fluid with GDT [Citation17,Citation19–Citation21].
5 Limitations of the study
This single-center design was a potential limitation of the trial, with a possible bias by institutional standards of care. Also, the enrollment of different surgical procedures might influence the results, considering variable pathophysiology and nature of complications between vascular and non-vascular abdominal surgery. Our goal was to study the hemodynamic optimization on major surgical population in our institution. Therefore, selection of more homogenous surgical population or studying subgroups of a larger sample could share in getting more significant results.
Our trial lacked power to show a significant reduction in mortality. Even though, the reduction in morbidity in small surgical population gave a remarkable value for this relatively simple intervention; however, it will need further evaluation in a larger multi-center study.
6 Conclusion
The applied program of intraoperative GDT for high-risk patients undergoing major abdominal surgeries provided satisfactory outcome with significant reduction of PO morbidities, need for blood products transfusion and ICU and hospital LOS. However, the applied intraoperative GDT program could not significantly reduce mortality rates.
Conflict of interest
None.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- T.W.ScheerenC.WiesenackH.GerlachG.MarxGoal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre studyJ Clin Monit Comput2732013225233
- Y.PiS.JinPerioperative fluid management in gastrointestinal surgeryZhonghua Wei Chang Wai Ke Za Zhi1872015642645
- A.FeldheiserP.ConroyT.BonomoB.CoxT.R.GarcesC.SpiesAnaesthesia working group of the Enhanced Recovery After Surgery (ERAS®) society; enhanced recovery after surgery society: development and feasibility study of an algorithm for intraoperative goaldirected haemodynamic management in noncardiac surgeryJ Int Med Res404201212271241
- C.Y.WuY.J.ChengY.J.LiuT.T.WuC.T.ChienK.C.ChanPredicting stroke volume and arterial pressure fluid responsiveness in liver cirrhosis patients using dynamic preload variables: A prospective study of diagnostic accuracyEur J Anaesthesiol3392016645652
- J.A.HoodR.J.WilsonPleth variability index to predict fluid responsiveness in colorectal surgeryAnesth Analg1135201110581063
- N.L.HolmeE.B.ReinM.ElstadCardiac stroke volume variability measured non-invasively by three methods for detection of central hypovolemia in healthy humansEur J Appl Physiol2016 2016 September 10 [Epub ahead of print]
- M.CannessonH.MusardO.DesebbeC.BoucauR.SimonR.HnaineThe ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patientsAnesth Analg10822009513517
- B.KhwannimitR.BhurayanontachaiPrediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variationEur J Anaesthesiol29220126469
- J.MayerJ.BoldtA.M.MengistuK.D.RohmS.SuttnerGoal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trialCrit Care1412010R18
- American Society of Anesthesiologists. ASA Physical Status Classification System Last approved by the ASA House of Delegates on October 15; 2014.
- T.H.LeeE.R.MarcantonioC.M.MangioneE.J.ThomasC.A.PolanczykE.F.CookDerivation and prospective validation of a simple index for prediction of cardiac risk of major non-cardiac surgeryCirculation10010199910431049
- K.WassermanW.L.BeaverJ.A.DaviesJ.Z.PuD.HeberB.J.WhippLactate, pyruvate and lactate-to-pyruvate ratio during exercise and recoveryJ Appl Physiol5931985935940
- W.D.NearyB.P.HeatherJ.J.EarnshawThe Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (POSSUM)Br J Surg902003157165
- H.C.KraemerS.TheimannHow many subjects? Statistical power analysis in research1987SageNewbury Park, CA
- K.R.MurphyB.MyorsStatistical power analysis: a simple and general model for traditional and modern hypothesis tests2nd ed.2003Lawrence Erlbaum Associates Inc.
- Q.LinH.ZhouD.LiJ.YeJ.HongY.HuEffect of perioperative goal-directed fluid therapy on clinical outcome in elective colorectal resectionZhonghua Wei Chang Wai Ke Za Zhi1872015671675
- S.L.LahtinenJ.H.LiisananttiM.M.PoukkanenP.A.LaurilaGoal-directed fluid management in free flap surgery for cancer of the head and neckMinerva Anestesiol2016 2016 October 19 [Epub ahead of print]
- G.R.ManeckeA.AsemotaF.MichardTackling the economic burden of postsurgical complications: would perioperative goal-directed fluid therapy help?Crit. Care1852014566
- J.L.MuñozT.GabaldónE.MirandaD.L.BerrioJ.Ruiz-TovarJ.M.RondaGoal-directed fluid therapy on laparoscopic sleeve gastrectomy in morbidly obese patientsObes Surg2611201626482653
- M.R.BacchinC.M.CeriaS.GiannoneD.GhisiG.StagniT.GreggiGoal-directed fluid therapy based on stroke volume variation in patients undergoing major spine surgery in the prone position: a cohort studySpine (Phila Pa 1976)41182016E1131E1137
- T.KratzC.SimonV.FendrichR.SchneiderH.WulfC.KratzImplementation and effects of pulse-contour- automated SVV/CI guided goal directed fluid therapy algorithm for the routine management of pancreatic surgery patientsTechnol Health Care2016 2016 July 8 [Epub ahead of print]
- J.BenesM.GiglioN.BrienzaF.MichardThe effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trialsCrit Care1852014584
- K.E.RollinsD.N.LoboIntraoperative goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trialsAnn Surg26332016465476
- A.SomS.MaitraS.BhattacharjeeD.K.BaidyaGoal directed fluid therapy decreases postoperative morbidity but not mortality in major non-cardiac surgery: a meta-analysis and trial sequential analysis of randomized controlled trialsJ Anesth31120176681
- O.KruseN.GrunnetC.BarfodBlood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic reviewScand J Trauma Resusc Emerg Med19201174
- J.BenesI.ChytraP.AltmannM.HluchyE.KasalR.SvitakIntraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized studyCrit Care1432010R118
- J.YuR.ZhengH.LinQ.ChenJ.ShaoD.WangGlobal end-diastolic volume index vs central venous pressure goal-directed fluid resuscitation for chronic obstructive pulmonary disease patients with septic shock: a randomized controlled trialAm J Emerg Med2016 pii: S0735–6757(16)30710–0
- C.W.LaiT.StarkieS.CreanorR.A.StruthersD.PortchP.D.ErasmusRandomized controlled trial of stroke volume optimization during elective major abdominal surgery in patients stratified by aerobic fitnessBr J Anaesth11542015578589
- S.SrinivasaM.H.TaylorP.P.SinghT.C.YuM.SoopA.G.HillRandomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomyBr J Surg100120136674
- S.SrinivasaM.H.TaylorP.P.SinghD.P.LemanuA.D.MacCormickA.G.HillGoal-directed fluid therapy in major elective rectal surgeryInt J Surg1212201414671472
- A.JoostenT.HuynhK.SuehiroC.CanalesM.CannessonJ.RinehartGoal-Directed fluid therapy with closed-loop assistance during moderate risk surgery using noninvasive cardiac output monitoring: A pilot studyBr J Anaesth11462015886892