Abstract
Objective
Debridement of burn wounds and skin graft harvesting is associated with increased peri-operative bleeding. In this study we evaluated the effectiveness of tranexamic acid in reducing blood transfusion requirements during burn wound debridement/eschar removal and skin graft harvesting in adults with major burn injuries, with the primary outcome being the total amount of intraoperative blood loss.
Methods
Fifty adult patients having >20% total body surface area of burn wounds, scheduled for wound debridement/eschar removal ± skin grafting after 10 days of burn injury under general anaesthesia were included. Patients were randomly allocated to receive either injection tranexamic acid 15 mg/kg diluted to 25 ml with isotonic saline over 10 min or an equal volume of only isotonic saline before induction of general anaesthesia. Venous blood gas analysis was done in the beginning and end of surgery, and then at 24 postoperative hours to assess hemoglobin levels of the patients. Blood transfusion was given when hemoglobin levels fell down to or below 7 gm/dl. Intraoperative blood loss was calculated using the Gross formula.
Results
Intraoperative blood loss was found to be significantly higher in placebo group compared to tranexamic group, 990 ± 358.9 ml vs 581 ± 333.2 ml (p < 0.00), with more blood and colloid solutions being used to replace the blood loss in placebo group (p < 0.05).
Conclusions
Preoperative administration of a single dose of tranexamic acid significantly reduces blood loss during debridement of burn wounds and skin graft harvesting surgeries without increasing the risk of untoward side-effects or complications.
1 Introduction
Debridement of burn wounds and skin graft harvesting is associated with increased peri-operative bleeding, particularly in cases where the percentage of total body surface area burnt is high. These surgeries maybe associated with blood loss significant enough to warrant transfusion of blood products [Citation1]. It is important that we use blood and blood components judiciously, since they are an expensive and a limited resource. Further, serious side-effects can occur following transfusion of these products, thus increasing perioperative morbidity and mortality. Hence, various blood conservation strategies including subcutaneous or topical adrenaline, topical thrombin, limb tourniquets have been used to reduce blood loss during burn wound debridement [Citation2–Citation4]. But, these techniques are not used consistently [Citation5] and search for cost-effective alternatives is still going on.
In recent years, role of antifibrinolytic agents (aprotinin, tranexamic acid and epsilon-aminocaproic acid) to reduce perioperative bleeding and thus the requirement of blood transfusion has been evaluated [Citation6]. Of the various antifibrinolytic agents available, the effectiveness and safety of tranexamic acid in reducing surgical blood loss has been widely studied in joint arthroplasties, cardiac surgical procedures, paediatric surgeries and in musculoskeletal trauma patients [Citation7–Citation12]. A number of previous reports [Citation13,Citation14] suggest various other techniques that are available to help reduce blood loss in burn surgeries. These include topical application of adrenaline soaks, thrombin solutions and subcutaneous adrenaline infiltration. However, there are no reports in literature evaluating the role of tranexamic acid in reducing surgical blood loss and blood transfusion requirements during burn wound debridement. Thus, the present study was planned with the aim of evaluating the effectiveness of tranexamic acid in reducing blood transfusion requirements during burn wound debridement/eschar removal and skin graft harvesting in adults with major burn injuries. Primary outcome measure of our study was the total amount of intraoperative blood loss and secondary outcome measures included blood transfusion requirements (transfusion trigger point of 7 gm/dl), the change in hemoglobin and hematocrit levels following burn wound debridement/eschar removal and skin graft harvesting as well as any untoward effects attributed to the use of study drug.
2 Methods
After institutional ethics committee approval, this prospective, randomized, double blind, placebo controlled study was carried out by the department of Anaesthesia & Intensive Care and Plastic Surgery over a period of 2 years (July 2013 to July 2015). After obtaining written informed patient consent, 50 adult patients of either sex in the age group of 18–50 years, ASA physical status I/II, having >20% total body surface area (TBSA) of burn wounds, scheduled for wound debridement/eschar removal ± skin grafting after 10 days of burn injury under general anaesthesia were included in the study. Only third degree burns patients with obvious eschar were taken for debridement. Patients with a documented history of infarction, unstable angina, renal or hepatic insufficiency, pregnancy, ocular pathology, coagulopathy and those with allergy to tranexamic acid were excluded from the study.
The study design was prospective, randomized, double blind and placebo controlled. Using a computer generated random number table, patients were randomly allocated to either tranexamic acid group (n = 25) or placebo group (n = 25). Allocation concealment was done using sequentially numbered coded sealed envelopes. The study drugs were prepared in identical looking syringes by independent investigator not involved in recording the observations. The contents of syringe were unknown to both the surgeon who was operating and the anaesthesiologist involved in administering the drug and recording of observations. Decoding was done on completion of the study.
All patients were kept fasting after midnight and pre-medicated with tablet alprazolam 0.25 mg and tablet ranitidine 150 mg orally night before and two hours prior to surgery. In the operating room, patients were monitored for heart rate (HR), non-invasive blood pressure (NIBP), continuous electrocardiogram (ECG), arterial oxygen saturation (SpO2), end tidal carbon dioxide (EtCO2) and temperature using multichannel monitors. Baseline readings were recorded and an intravenous access was established in all patients. Tranexamic acid group patients (n = 25) received injection tranexamic acid 15 mg/kg diluted to 25 ml with isotonic saline over 10 min and placebo group patients (n = 25) received an equal volume of only isotonic saline before induction of general anaesthesia.
A standard technique was used for general anaesthesia induction in all the groups. Anaesthesia was induced with intravenous Morphine 0.1 mg kg−1 and Propofol 2–3 mg kg−1. Vecuronium bromide 0.1 mg kg−1 was used to facilitate tracheal intubation. Maintenance of anaesthesia was provided with 66% nitrous oxide in oxygen supplemented with isoflurane (1–2%). Venous blood gas analysis was done in the beginning of surgery, at the end of procedure and then at 24 postoperative hours to assess the hemoglobin levels of the patients. Blood transfusion was given to the patients in cases where the hemoglobin levels fell down to or below 7 gm/dl. In all patients in our study we also used adrenaline soaked (1:2,00,000 dilution) gauze pieces alongwith cautery and limb elevation to decrease amount of blood loss. At the end of surgery, all patients received ondansetron 4 mg i.v. and residual neuromuscular blockade was reversed with intravenous neostigmine 50 µg kg−1 and glycopyrrolate 10 µg kg−1.
Intraoperative blood loss was calculated using the formula described by Gross [Citation15]: CBL = EBV × [(Hb (i) – Hb (f))/Hb (m)] + Tx where CBL is the calculated blood loss, EBV is the estimated blood volume, Hb (i), Hb (f) and Hb (m) are the initial, final and mean (of initial and final) hemoglobin levels respectively and Tx is the total transfusion volume received (in milliliters). Any complications or side-effects due to the study drugs were also recorded.
The data was analyzed with Statistical Package for Social Studies (SPSS for windows 14, Chicago, IL, USA). Patient characteristics were analyzed by the Chi-square test for nominal data. Parametric data (age, height, weight) was analyzed using the independent samples t-test. Non parametric data was analyzed using Mann Whitney-U test. To evaluate anaesthetic data the independent samples t-test or the Mann Whitney-U test was used. Quantitative data was expressed as mean ± S.D. Categorical data was expressed as median (IQR) or number (%). P-value of <0.05 was considered statistically significant.
As this was the first study evaluating the effect of tranexamic acid in burn patients, we did pilot cases (10 patients in each group) to determine the sample size. Mean blood loss was 900 ± 200 ml in placebo group and 700 ± 200 ml in tranexamic acid group. To detect this difference with 90% power and a level of significance of 5%, we needed to recruit 22 patients in each group. To account for possible dropouts we decided to include 25 patients in each group.
3 Results
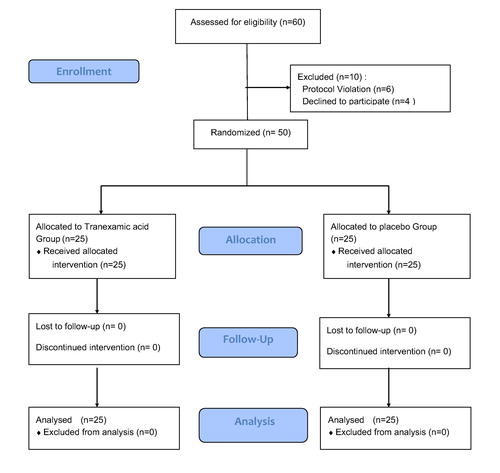
Sixty patients were assessed for eligibility, of which 6 patients did not meet the inclusion criteria and 4 patients refused to give consent for participation in the study. A total of fifty patients were included in the study and eventually analyzed. The CONSORT flow diagram is presented in .
Both the groups were comparable in terms of patient demographics (i.e., age, weight, gender distribution, patient temperature). Total body surface area burnt (%), duration of surgery and number of patients undergoing limb debridement and trunk debridement was also comparable between the two groups (). In both the group of patients, on an average only 15–20% of the total burnt area was debrided, with skin grafting being done in 8 patients in tranexamic group and 6 patients in placebo group.
Table 1 Demographic characteristics of patients.
Intraoperative blood loss per percentage total burn surface area was significantly higher in placebo group as compared to tranexamic group (), with the mean blood loss being 990 ± 358.9 ml vs. 581 ± 333.2 ml (p < 0.00) in the placebo and tranexamic groups respectively.
Table 2 Intraoperative blood loss in both groups.
Preoperative hematological status (hemoglobin and hematocrit) was comparable between the two groups (). However, as compared to the tranexamic group, in the placebo group, postoperative hemoglobin, immediately and at 24 postoperative hours, was significantly lower (p < 0.05). The immediate postoperative hematocrit was also significantly lower in placebo group as compared to tranexamic group ().
Table 3 Preoperative hematologic data.
Table 4 Postoperative hematologic data.
In the placebo group more blood and colloid solutions were used to replace the blood loss (p < 0.05) (). In tranexamic group, only 6 patients required blood transfusion compared to 13 patients in placebo group (p < 0.05) and only 7 units of blood was transfused in tranexamic group while a total of 20 units of blood was transfused in placebo group.
Table 5 Perioperative replacement solutions used in both groups.
None of our patients had any complications or side-effects with the use of tranexamic acid.
4 Discussion
Burn wound debridement involves removal of necrotic and devitalized tissue from the wound so as to obtain a vascularized wound bed which promotes healing and prevents infection. Debridement also facilitates survival of skin grafts. However, one of the main concerns with this procedure is the increased blood loss from donor as well as debridement sites, thus resulting in greater requirement of blood transfusions, along with its potential complications like high risk of bacterial infection and increased costs [Citation16]. Though, at times, it is rather difficult to control blood loss, but it is often beneficial to use strategies that minimize blood loss and thus reduce patient morbidity and mortality [Citation17].
Reported incidence of blood loss in extensive escharectomy and microskin graft placement is 77. 29 ml per 1% TBSA [Citation18]. Burn wound excision is associated with more blood loss, with 3.5–5% of the blood volume being lost for every 1% of the body surface area excised and grafted [Citation19,Citation20]. Previous studies have evaluated various techniques for reducing blood loss, including manual compression, use of tourniquet, topical and subcutaneous epinephrine, topical thrombin and fibrin glue [Citation17,Citation21,Citation22]. However, efficacy of these techniques is not well defined.
Primary hyperfibrinolysis is one of the main reasons for intraoperative blood loss that occurs during surgery. This forms the basis for the use of antifibrinolytic agents, like aprotonin and tranexamic acid, to reduce perioperative blood loss and transfusion requirements [Citation23]. Aprotonin is an expensive medication and its administration can result in anaphylaxis, rhabdomyolysis, obstructive uropathy, and myoglobinuria. Tranexamic acid is a cost-effective synthetic antifibrinolytic drug. It competitively decreases the activation of plasminogen to plasmin [Citation24] and has been successfully used in the past for decreasing perioperative blood loss in cardiac, spine, maxillofacial surgeries, total knee and hip arthroplasties, neurosurgeries, gynecologic surgeries and cesarean sections [Citation25–Citation28]. In a recent retrospective analysis, Dominguez et al. [Citation29] evaluated transfusion requirements in severely burnt patients. They concluded that the incidence of allogenic blood transfusion significantly reduced following intraoperative tranexamic acid administration. However, till date the effectiveness of tranexamic acid in burn surgeries has never been prospectively evaluated. We studied its role in burn wound debridement and skin graft harvesting surgeries in patients having >20% TBSA of burn wounds. It was observed that with the intravenous administration of 15 mg/kg tranexamic acid there was a significant reduction in intraoperative blood loss, with significantly less number of patients requiring blood transfusion. Postoperative hemoglobin and haematocrit levels were also higher in patients receiving tranexamic acid preoperatively.
We, in our study, included patients coming for burn wound debridement and graft harvesting after 10 days of burn injury, as debridements are often done at this stage. Early burn wound excision is not a very frequently performed procedure at our institute, as excisions, especially tangential, are associated with significant amount of blood loss. Though debridement at 2 weeks following burn injury is associated with less blood loss as compared to burn wound tangential excision, however these losses are significant enough to greatly increase patient morbidity. Bleeding increases the patient’s hospital stay, may necessitate re-operation, increases skin graft loss as well as the need for blood transfusion. Further, there is increased risk of hemolytic reactions, anaphylaxis, infections and acute lung injury with blood transfusions. Thus measures that significantly decrease blood loss greatly improve patient outcome.
The pre-incisional administration of tranexamic acid is known to decrease bleeding in total hip arthroplasty, knee arthroplasty and cesarean delivery. Tranexamic acid is known to be most effective when it is administered prophylactically or preoperatively, with it having little benefit when given intraoperatively [Citation30,Citation31]. This is because surgical procedure is associated with fibrinolytic activation, which is most easily inhibited in its earlier phase. Thus for its optimum effect, tranexamic acid should be administered prophylactically at an earlier stage [Citation30,Citation32]. We, in our study also administered tranexamic acid prophylactically, prior to start of surgical procedure and found it to be highly beneficial in reducing blood loss and blood transfusion requirements.
We did not find any side-effects or complications like hypotension, allergic reactions, postoperative nausea, vomiting, headache, dizziness, infection, thromboembolic episodes with the use of tranexamic acid in our study. As is well known, tranexamic acid should be administered slowly as an infusion, as its rapid administration is known to cause hypotension. Although a recent review has reported the incidence of venous thromboembolic events to be to the tune of 0.7%, however, many previous studies have also confirmed that the use of tranexamic acid does not increase the risk of thrombotic complications [Citation33]. Nonetheless, it should be used with caution in patients with history of a thromboembolic event or a family history of thromboembolic disease.
One of the main limitation of our study was that our sample size was not adequately sized to comment on the long term benefits of administering tranexamic acid like 6 months or 1 year mortality rate, graft failure rate, renal failure rate, etc. Further studies, with larger sample size need to be conducted in future to find out the long term clinical benefits of administering tranexamic acid.
5 Conclusion
Preoperative administration of a single dose of tranexamic acid significantly reduces blood loss during debridement of burn wounds and skin graft harvesting surgeries without increasing the risk of untoward side-effects or complications. Therefore, the cost-benefit ratio of single dose tranexamic acid therapy seems to be extremely rewarding as it significantly decreases perioperative blood transfusion requirements and its associated risks.
Conflict of interest
None.
Funding
None.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- K.LangleyK.SimAnaesthesia for patients with burn injuriesCurr Anaesth Crit Care1320007075
- B.S.BrezelK.E.McGeeverJ.M.SteinEpinephrine v thrombin for split-thickness donor site hemostasisJ Burn Care Rehabil81987132134
- A.C.KiserC.W.LentzH.D.PetersonSubcutaneous injection of donor sites for split-thickness skin graftsJ Am Coll Surg1821996265267
- T.JanezicB.PrezeljA.BrcicZ.ArnezL.Zaletelj-KrageljIntraoperative blood loss after tangential excision of burn wounds treated by subeschar infiltration of epinephrineScand J Plast Reconstr Surg Hand Surg311997245250
- R.CartottoM.A.MusgraveM.BeveridgeJ.FishM.GomezMinimizing blood loss in burn surgeryJ Trauma49200010341039
- I.RobertsH.ShakurK.KerT.CoatsCRASH-2 Trial collaborators. Antifibrinolytic drugs for acute traumatic injuryCochrane Database Syst Rev192011CD004896
- S.M.GoobieP.M.MeierL.M.PereiraF.X.McGowanR.P.PrescillaL.A.ScharpEfficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trialAnesthesiology1142011862871
- P.N.KakarN.GuptaP.GovilV.ShahEfficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trialIndian J Anaesth532009667671
- S.ThiagarajamurthyA.LevineJ.DunningDoes prophylactic Tranexamic acid safely reduce bleeding without increasing thrombotic complications in patients undergoing cardiac surgery?Interact Cardiovasc Thorac Surg32004489494
- H.FawzyE.ElmistekawyD.BonneauD.LatterL.ErrettCan local application of tranexamic acid reduce post-coronary bypass surgery blood loss? A randomized controlled trialJ Cardiothorac Surg4200925
- S.ElwatidyZ.JamjoomE.ElgamalA.ZakariaA.TurkistaniA.El-DawlatlyEfficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled studySpine33200825772580
- W.S.ChoiM.G.IrwinN.SammanThe effect of tranexamic acid on blood loss during orthognathic surgery: a randomized controlled trialJ Oral Maxillofac Surg672009125133
- A.H.RobertsThe effect of topical epinephrine on blood loss following tangential excision of burn woundsPlast Reconstr Surg741984450451
- C.F.SnellingK.ShawThe effect of topical epinephrine hydrochloride in saline on blood loss following tangential excision of burn woundsPlast Reconstr Surg721983830836
- J.B.GrossEstimating allowable blood loss: correcting for dilutionAnesthesiology581983277280
- R.L.SheridanS.K.SzyfelbeinStaged high-dose epinephrine clysis is safe and effective in extensive tangential burn excisions in childrenBurns251999745748
- J.P.BarretP.DziewulskiS.E.WolfM.H.DesaiR.J.NicholsD.N.HerndonEffect of topical and subcutaneous epinephrine in combination with topical thrombin in blood loss during immediate near-total burn wound excision in pediatric burned patientsBurns251999509513
- G.LuoH.FanW.SunY.PengL.ChenJ.TaoBlood loss during extensive escharectomy and auto-microskin grafting in adult male major burn patientsBurns3752011790793
- P.G.BudnyP.J.ReganA.H.RobertsThe estimation of blood loss during burns surgeryBurns191993134137
- T.A.HousingerD.LangG.D.WardenA prospective study of blood loss with excisional therapy in pediatric burn patientsJ Trauma341993262263
- F.A.OfodileM.K.SadanaThe role of topical thrombin in skin graftingJ Natl Med Assoc831991416418
- B.M.AchauerS.R.MillerT.E.LeeThe hemostatic effect of fibrin glue on graft donor sitesJ Burn Care Rehabil1519942428
- M.VerstraeteClinical application of inhibitors of fibrinolysisDrugs291985236261
- P.D.MonganR.S.BrownB.G.ThwaitesTranexamic acid and aprotinin reduce postoperative bleeding transfusions during primary coronary revascularizationAnesth Analg871998258265
- C.J.DunnK.L.GoaTranexamic acid: a review of its use in surgery and other indicationsDrugs57199910051032
- N.CelebiB.CelebiogluM.SelcukO.CanbayA.H.KaragozU.AyparThe role of antifibrinolytic agents in gynecological cancer surgerySaudi Med J272006637641
- E.LemayJ.GuayC.CoteA.RoyTranexamic acid reduces the need for allogenic red blood cell transfusions in patients undergoing total hip replacementCan J Anesth5120043137
- C.C.WuW.N.HoS.B.ChengD.C.YehWen.McT.J.LiuPerioperative parenteral tranexamic acid in liver tumor resectionAnn Surg2432006173180
- A.DominguezE.AlsinaL.LandinJ.F.Garcia-MiguelC.CasadoF.GilsanzTransfusion requirements in burn patients undergoing primary wound excision: effect of tranexamic acidMinerva Anestesiol.8342017353360
- D.AmarF.M.GrantH.ZhangP.J.BolandD.H.LeungJ.A.HealeyAntifibrinolytic therapy and perioperative blood loss in cancer patients undergoing major orthopedic surgeryAnesthesiology982003337342
- N.TanakaH.SakahashiE.SatoK.HiroseT.IshimaS.IshiiTiming of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the kneeJ Bone Joint Surg Br832001702705
- W.G.MurphyM.J.DaviesA.EduardoThe haemostatic response to surgery and traumaBr J Anaesth701993205213
- Q.WangJ.LiuR.FanY.ChenH.YuY.BiTranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trialEur Spine J22201320352038