Abstract
Objective
To compare the use of rocuronium with that of dexmedetomidine as an adjuvant to the local anesthetic mixture in peribulbar anesthesia for cataract surgery.
Design
A double blind, prospective, randomized controlled study.
Methods
Ninety patients with cataract in the age group 18–80 years of American Society of Anesthesiologists (ASA) physical status I–III scheduled for elective cataract surgery under regional anesthesia were randomly divided into three groups; Group C (control) received peribulbar anesthesia using a mixture of 4 ml lidocaine 2%, 4 ml bupivacaine 0.5%, and 1 ml normal saline. Group R received a mixture of 4 ml lidocaine 2%, 4 ml bupivacaine 0.5%, and 0.06 mg/kg rocuronium (maximum 5 mg) in 1 ml saline. Group D received a mixture of 4 ml lidocaine 2%, 4 ml bupivacaine 0.5%, and dexmedetomidine 50 μg (1 ml). Patients were assessed for onset and duration of corneal anesthesia and globe akinesia, postoperative pain using visual analog score (VAS), intraocular pressure (IOP), and sedation level using modified Ramsay sedation score (RSS). Patient and surgeon satisfaction score were also assessed.
Results
Corneal anesthesia was achieved more rapidly in groups D and R than group C (P < 0.01). Akinesia was achieved more rapidly in the group R than both group D and group C. Akinesia was achieved more rapidly in group D than the control group. Intraocular Pressure was significantly lower in group D compared to both the control group and group R. Ramsay sedation score was significantly higher in group D compared to both the control group and D. Patient and surgeon satisfaction was significantly higher in group R and D compared to the control group.
Conclusion
Adding 5 mg rocuronium to local anesthetic mixture provides more rapid onset of corneal and globe akinesia than 50 μg dexmedetomidine. Adding dexmedetomidine decreases IOP and provides sedation.
1 Introduction
With the introduction of safer local anesthetics, and the desire to mobilize the patient in the early postoperative period, local anesthesia has become more popular in ophthalmic surgery. Peribulbar anesthesia is widely used for cataract surgery. This technique is associated with fewer serious complications compared with retrobulbar anesthesia [Citation1]. However, it has the disadvantages of a slow onset of orbital akinesia [Citation2] and the frequent need for block supplementation [Citation3]. To overcome these limitations, many adjuvant drugs such as adrenaline, sodium bicarbonate, hyaluronidase, clonidine, and opioids [Citation4–Citation8] have been added to the local anesthetic mixture used for peribulbar block to augment its efficacy and hasten its speed of onset; however, their effects have been variable. Neuromuscular blocking drugs, such as vecuronium [Citation9] and atracurium [Citation10], have also been added to the local anesthetic mixture and have been shown to improve the quality of peribulbar anesthesia. Atracurium, however, has histamine-releasing properties and could result in undesirable local hyperemia. In contrast, rocuronium is devoid of this adverse effect and has a faster onset of action; its effects in a low dose on the quality of peribulbar anesthesia; onset time and need for supplemental injection of local anesthetics, have not been fully explored.
Dexmedetomidine is a centrally acting, highly specific α2-agonist. It has dose dependant sedative, anxiolytic, analgesic, and sympatholitic actions without causing relevant respiratory depression. Dexmedetomidine has been used as an additive to local anesthetics in peripheral nerve block[Citation11], brachial plexus block [Citation12], subarachnoidanesthesia [Citation13] and peribulbar anesthesia [Citation14,Citation15] to shorten the onset and prolong the duration of analgesia.
The aim of this study is to compare the use of rocuronium with that of dexmedetomidine as an adjuvant to the local anesthetic mixture in peribulbar anesthesia for cataract surgery.
2 Methodology
The study was conducted in Magrabi Specialist Eye Center, Dammam, Saudi Arabia between September 2015 and April 2016. The Institutional Review Board (IRB) of Magrabi Eye Center was responsible for the review and approval of the study protocol before patient enrollment, taking into consideration the legislative requirements and existing national regulations, and written informed consent was obtained from all patients. Patients aged between 18 and 80 years of American Society of Anesthesiologists (ASA) physical status I-III scheduled for cataract surgery using phacoemulsification technique with intraocular lens implantation by the same surgeon were enrolled in this double blind, prospective, randomized controlled study. Patients were excluded from the study if they were known to have severe uncontrolled hypertension, orthopnea, abnormal bleeding tendencies, difficulty in communication, extraocular muscle or eyelid abnormalities, allergy to any of the study drugs, high myopia (axial length >26 mm), single eye, ocular infection, or mental retardation.
Preoperative investigations such as evaluation of the complete blood picture, coagulation profile, metabolic profile, Electrocardiogram (ECG), and chest radiograph were carried out when appropriate. Details of the anesthetic technique and the study protocol were explained to the patients at their preoperative visit. Patients were explained about the procedure involved in the peribulbar block and the use of Visual Analog Scale (VAS) of 10 cm to evaluate the pain perceived by them; zero cm representing no pain and 10 cm representing the worst pain imaginable. An intravenous line was inserted and patients were sedated with intravenous (IV) midazolam (0.02 mg/kg) 5 min before the block in the preparation room, then they were transferred to the operating room and attached to the standard monitors (ECG, peripheral oxygen saturation and noninvasive blood pressure) and oxygen (2 L/min) was administered through nasal prongs.
Using a computer-generated randomization schedule and serially numbered, opaque, sealed envelopes, patients were randomly allocated to one of three study groups. Group C received peribulbar anesthesia using a mixture of 4 ml lidocaine 2%, 4 ml bupivacaine 0.5%, and 1 ml normal saline. Group R received a mixture of 4 ml lidocaine 2%, 4 ml bupivacaine 0.5%, and 0.06 mg/kg rocuronium (maximum 5 mg) in 1 ml saline. Group D received a mixture of 4 ml lidocaine 2%, 4 ml bupivacaine 0.5%, and dexmedetomidine 50 μg (1 ml). Medications were prepared in the pharmacy in a 10 ml syringe labeled as “study drug” to maintain blinding. All physicians, patients, nursing staff, and data collectors were blinded to the patient group assignment. Topical anesthesia of the conjunctiva and cornea was provided by administering two to three drops of 0.4% benoxinate hydrochloride. After sterilization, 4.5 ml of the study solution was injected into each of the following two sites: inferotemporal and medial canthus peribulbar areas, separated by an adequate gentle orbital massage for at least 40 s. The injection was administered using a 25G short bevel needle (25 mm in length) [Citation16].
A Honan’s cuff was immediately applied to the eye afterward and inflated to 30 mmHg for a total of 10 min. During this time, the cuff was deflated every 2 min to assess ocular movements and the orbicular muscle. Corneal anesthesia was also evaluated using a small cotton wool at the same time intervals. Motor block evaluation included; evaluation of lid akinesia (lid closure and squeezing by orbicularis and lid opening by the levator palpebrae muscle). For assessment of lid akinesia, patients were asked to open their eyelids and then squeeze them together maximally using a three-point scale (0–2) in which 0 refers to complete akinesia; 1 refers to partial movement in either or both eyelid margins; 2 refers to normal movement in either or both eyelid margins. Evaluation of globe akinesia was achieved using a three-point scale (0–2) for each of the four cardinal directions (upward, downward, nasal, and temporal). Ocular movement in each direction was scored as 2 if it was normal, 1 if it was limited, and 0 if there was no directional movement[Citation17] (total score of 8).
A total score of 10 is obtained when we added the globe akinesia score (0–8) and the lid akinesia score (0–2). Arterial blood pressure, heart rate, and oxygen saturation (SpO2) were recorded every 15 min during the entire procedure and every 30 min during the first two postoperative hours. Hypotension and bradycardia were defined as a 20% decrease in blood pressure and heart rate in relation to the pre-block value. Time to the adequate condition to begin surgery (defined as the presence of corneal anesthesia together with an ocular movement score ≤1 in every direction and an eyelid akinesia score of 0) was recorded using a stopwatch. If the adequate condition to begin surgery was not achieved 10 min after performing the block, a supplemental injection of 3 ml of 2% lidocaine was administered either inferotemporally or medially on the basis of the anesthesiologist's assessment.
Additionally, Intra-ocular pressure (IOP) was measured by the anesthesiologist using a hand-held applanation tonometer, before injection oflocal anesthetic (baseline) and after complete akinesia of the globe before surgical incision. Sedation levels were assessed using a modified Ramsay Sedation Scale (RSS) [Citation18] (1, anxious and agitated or restless, or both; 2, cooperative, oriented, and tranquil; 3, responds to commands only; 4, brisk response to light glabellar tap or loud auditory stimulus; 5, sluggish response to light glabellar tap or loud auditory stimulus 6, no response to light glabellar tap or loud auditory stimulus) every 15 min during the surgery and for the first hour thereafter.
At the end of surgery, all patients were asked to rate their intraoperative pain using a Visual Analogue Scale (VAS) with two anchor points; 0 being no pain and 10 being the worst imaginable pain. Postoperatively, both the surgeon and the patients were asked to assess their satisfaction on a five-point Likert scale, where 5 indicated very satisfied, 4 indicated satisfied, 3 indicated neutral, 2 indicated unsatisfied, and 1 indicated very unsatisfied.
3 Statistical methods
The required sample size was calculated using G∗Power software version 3.1.0 (Institutfür Experimentelle Psychologie, Heinrich Heine Universität, Düsseldorf, Germany). The primary outcome measure was the akinesia score. Assuming a type I error of 0.05, it was estimated that a sample size of 25 patients in each study group would achieve a power of 87% to detect an effect size (d) of 0.4 in the primary outcome of interest. Statistical analysis was carried out on a personal computer using IBMSPSS Statistics version 21 (IBMCorp., Armonk, New York, USA). Normality of numerical data distribution was tested using the Shapiro-Wilk test. Normally distributed numerical data were presented as mean and Standard Deviation (SD) or median and interquartile range, and intergroup differences were compared using one-wayanalysis of variance. The Tukey post hoc test was used for pairwise comparisons whenever a statistically significant test was detected on one-way analysis of variance. Categorical data were presented as number and percentage and intergroup differences were compared using the Pearson X2-test or Fisher’s exact test when appropriate. All P-values are two-tailed. A P-value of less than 0.05 was considered statistically significant.
4 Results
A hundred and one patients met the inclusion criteria and were approached for participation in the study; however, only ninety gave written informed consent to participate, were randomized in equal numbers to three study groups and were analyzed in their respective group, and had no protocol violations (). Patients’ characteristics were comparable among the three groups, as well as the duration of surgery, intraoperative VAS scores and time to onset of postoperative pain. The time to adequate conditions to start surgery was significantly lower in the rocuronium group and the dexemedetomidine compared to the control group and in the rocuronium compared to the dexmedetomidine group (P = 0.01). The need for supplemental injection was lower in the rocuronium and the dexemedetomidine group compared to the control group, however the difference did not reach statistical significance ().
Table 1 Patient characteristics.
Corneal anesthesia was achieved at 2 min after block administration in over twothirds of the patients in groups D and R compared with only a third of those in group C (P < 0.01). However, this difference disappeared by 4 min, and by 8 min all study patients except for one in group C had achieved corneal anesthesia ().
Table 2 Corneal Anesthesia.
Akinesia was achieved more rapidly in the rocuronium group than both the dexmeditomidine group and the control group at 2, 4, 6, 8 and 10 min. Additionally, akinesia was achieved more rapidly in the dexmeditomidine group than the control group at 4 and 8 min ().
Table 3 Akinesia score.
Baseline IOP was comparable among the three groups. However, IOP was found to be significantly lower in the dexmedetomidine group compared to both the control and the rocuronium groups after complete akinesia of the globe and before surgical incision. ().
Table 4 Comparison among groups regarding intra-ocular pressure (mmHg).
The median Ramsay Sedation Score (RSS) was found to be significantly higher in the dexmedetomidine group compared to both the control and the rocuronium groups at 15, 30, 45, min after injection ().
Table 5 Comparison among groups regarding Ramsay sedation score.
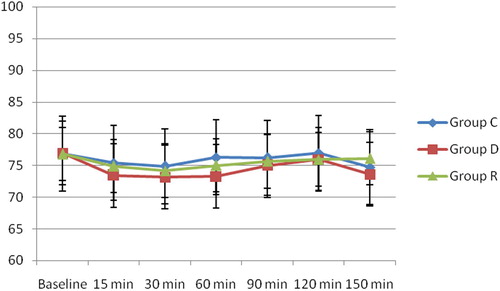
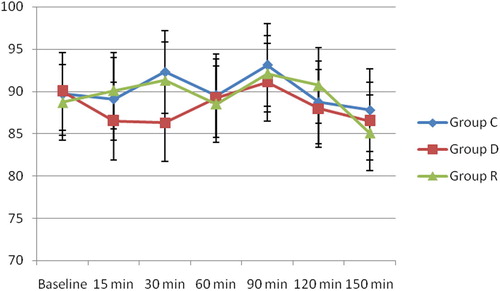
There was no statistically significant difference in the mean HR among the three groups (). There was no statistically significant difference in the Mean arterial blood pressure among the three groups, although it was slightly lower in group D than in group C and group R at 15 and 30 min after the block ().
The number of satisfied patients and surgeons was significantly higher in the rocuronium and the dexmedetomidine groups compared with the control group. Although the number of satisfied surgeons was higher in the rocuronium group than the dexmedetomidine group however this was found to be statistically non-significant ().
Table 6 Patient and surgeon satisfaction.
5 Discussion
The current study compared the effect of adding a neuromuscular blocking agent (rocuronium) and a centrally acting α2-agonist (dexmedetomidine) to a local anesthetic mixture (bupivacaine/lidocaine).
Several studies have compared the effect of adding different types of additives to improve the quality of block in ophthalmic local anesthesia with varying results [Citation4–Citation8] Adding neuromuscular blockers to the local anesthetic does not affect analgesia but induces akinesia in extraocular muscles which helps optimize the setting for ophthalmic surgery. The dose of rocuronium chosen in this study was less than one-tenth the dose administered intravenously for clinical neuromuscular blockade. Since this dose is used frequently as a priming dose, it can be considered safe [Citation19]. The exact mechanism through which the local administration of non-depolarizing muscle relaxants improves orbital akinesia is not fully known, but it has been hypothesized that they may have a topical action on ocular muscle motor neurons or that they may modulate muscle spindle activity thus decreasing muscle tone. Unintentional intravenous injection of a mixture containing a neuromuscular blocker or systemic absorption of rocuronium following peribulbar injection is a potential risk which may lead to muscle weakness.
With the introduction of phacoemulsification techniques, it is currently possible for cataract surgery to be successfully performed using topical anesthesia, reducing the clinical relevance of complete motor block. However, many ophthalmic surgeons prefer to operate on immobile eyes, and akinesia still remains of interest for many ophthalmic surgical procedures. In addition, a recent study suggested that patients prefer peribulbar block to topical anesthesia during cataract surgery [Citation20].
Dexmedetomidine is a highly specific centrally acting α2-agonist commonly used as sedative, preemptive analgesic [Citation21] and to maintain stable hemodynamics in laparoscopic surgeries [Citation22]. It can also be added to the local anesthetic mixture in peripheral nerve block [Citation11], brachial plexus block [Citation12], subarachnoid anesthesia [Citation13], and ophthalmic anesthesia [Citation14,Citation15].
The process by which α2-adregenic receptor agonists cause sedation and analgesia is not fully understood but it is most likely multi-factorial. α2-agonists produce analgesia peripherally by decreasing the release of norepinephrine and also by an α2-receptor-independent inhibitory effect on nerve fiber action potential. They produce analgesia centrally, by inhibiting the release of substance P in the nociceptive pathway at the level of the dorsal root neuron as well as by activation of α2 adrenoceptors in the locus coeruleus [Citation23,Citation24].
In this study, the onset of akinesia was more rapid in the rocuronium group (Group R) compared to the control group (Group C) and the dexmedetomidine group (Group D). There was also a significant difference between the dexmedetomidine group and the control group in favor of the dexmedetomidine group leading to less delay in surgery and more suitable conditions to operate in less than 10 min. In addition, the time to adequate conditions to start surgery was less in the rocuronium group. The rocuronium group needed a lesser dose of supplemental injection, reducing the possibility of complications that may be attributed to repeated injection such as globe perforation and hemorrhage, although the difference did not reach statistical significance.
Several authors have proposed that adding a low dose of neuromuscular blocker to a local anesthetic mixture in peribulbar block provides superior akinesia. The study by Aissaoui et al. [Citation25] demonstrated that the addition of rocuronium to a local anesthetic mixture in peribulbar block provides good akinesia and reduces the need for supplementary injections of local anesthetic. Similarly, Abdellatif et al. [Citation26] added a low dose of rocuronium to two different concentrations of local anesthetic mixture and concluded that a mixture of rocuronium 5 mg, lidocaine 2%, and bupivacaine 0.5% provides optimal orbital and eyelid akinesia for cataract surgery and shortens the block onset time. Again, Hamawy and Bestarous [Citation27] compared the addition of rocuronium or magnesium sulfate to the local anesthetic mixture (bupivacaine/lidocaine/hyaluronidase) and concluded that adding rocuronium to the local anesthetic mixture results in a better akinesia score and faster establishment of suitable conditions to start cataract surgery compared with the addition of magnesium to the same mixture. Additionally, Reah et al. [Citation9] added 0.5 mg vecuronium to a mixture of bupivacaine, lidocaine and 150 IU hyaluronidase. The findings of their study showed that the addition of vecuronium improves lid and globe akinesia without side effects. Similarly, Kucukyavuz and Arici [Citation10] added 5 mg atracurium to a mixture of bupivacaine-lidocaine without hyaluronidase and obtained comparable results.
Several studies have explored the effect of dexmedetomidine as an adjunct to local anesthesia of the eye. Channabasappa et al. [Citation28] reported that a combination of bupivacaine and lidocaine with dexmedetomidine in peribulbar anesthesia provides a degree of sedation that enables full cooperation. The authors also found that the mixture also helped to significantly decrease the IOP, shorten the onset time of motor and sensory block and extend their duration. Abdelhamid et al. [Citation14] compared the effect of 50 µg of dexmedetomidine added to the local anesthetic mixture or the use of an IV infusion of dexmedetomidine1 µg/kg and concluded that, as an additive, dexmedetomidine shortens the onset time, prolongs the block duration and significantly decreases the IOP with minimal side effects.
In the current study IOP was significantly lower in the dexmedetomidine group than the rocuronium group and the control group. Dexmedetomidine may decrease IOP by vasoconstriction of the afferent blood vessels in the ciliary body. This leads to decrease in production of aqueous humor [Citation29]. It may also facilitate drainage of aqueous humor by reducing the sympathetic-mediated vasomotor tone of the drainage system of the eye [Citation30]. Also, the hemodynamic effects of dexmedetomidine may play a role in the reduction of IOP [Citation31].
These results are similar to those obtained by Channabasappa et al. [Citation28] who reported that a combination of bupivacaine and lidocaine with dexmedetomidine in peribulbar anesthesia helped to decrease the IOP significantly. Furthermore, Abdelhamid et al. [Citation14] reported a significant decrease in IOP when adding dexmedetomidine 50 μg to a bupivacaine 0.5%-lidocaine 2%-hyaluronidase mixture compared to the control group which received the same mixture without dexmedetomidine. Similar results were also obtained by El-Ozairy and Tharwat [Citation15] who investigated the effect of adding two different doses of dexmedetomidine to a local anesthetic mixture of levobupivacaine and hyaluronidase in patients undergoing vitreoretinal surgery. The authors found the IOP to be significantly less in the dexmedetomidine groups at 1, 5, and 10 min after injection (P < 0.001). Madan et al. [Citation32] also found similar results when they added clonidine to lidocaine during cataract surgery performed under peribulbar block.
In this study, RSS was found to be significantly higher in the dexmedetomidine group than the control group and the rocuronium group at 15, 30, and 45 min after injection. Similar results were obtained by El-Ozairy and Tharwat [Citation15] who investigated the effect of adding two different doses of dexmedetomidine to a local anesthetic mixture of levobupivacaine/hyaluronidase and found the RSS to be significantly higher in groups receiving 25 and 50 µg of dexmedetomidine added to the local anesthetic mixture during the first 60 min following injection compared with the control group. The authors reported that these sedation scores allowed full patient cooperation during surgery. Comparable results were also reported by Channabasappa et al. [Citation28] who reported that a combination of bupivacaine and lidocaine with dexmedetomidine in peribulbar anesthesia provides sedation that enables full cooperation during cataract surgery. Additionally, the study showed that there was higher patient and surgeon satisfaction in the dexmedetomidine group and the rocuronium group compared to the control group.
6 Conclusion
The results of the present study have shown that the addition of 5 mg of rocuronium to lidocaine/bupivacaine mixture significantly shortened the onset of peribulbar block in patients undergoing cataract surgery compared to dexmedetomidine or placebo. The study also demonstrated that the addition of 50 μg dexmedetomidine to the same local anesthetic mixture lowered the IOP and provided sedation and patient cooperation.
Conflict of interest
The authors declared that there is no conflict of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- A.P.RubinComplications of local anesthesia for ophthalmic surgeryBr. J. Anaesth.7519959396
- D.H.WongRegional anesthesia for intraocular surgeryCan. J. Anaesth.401993635657
- J.H.LootsA.S.KoortsJ.A.VenterPeribulbar anesthesia. A prospective statistical analysis of the efficacy and predictability of bupivacaine and a lignocaine/bupivacaine mixtureJ. Cataract Refract. Surg.1919937276
- R.GottipatiR.K.AmarnathD.S.Deepraj Singh BaisR.RaoK.S.NanduPeribulbar block: a clinical comparision between clonidine and dexmedetomidine as adjuvants with lignocaine, bupivacaine mixtureJ. Evidence Based Med Healthc.39201562816290
- I.KamelA.MounirA.Z.FouadH.MekawyBakery E.EhabComparing different fentanyl concentrations added to local anesthetic mixture in peribulbar block for cataract surgeryEgypt. J. Anaesth.322016189193
- P.J.SarvelaComparison of regional ophthalmic anesthesia produced by pH-adjusted 0.75% and 0.5% bupivacaine and peribulbar 1% and 1.5% etidocaine, all with hyaluronidaseAnesth. Analg.771993131134
- K.ZahlA.JordanJ.McGroartyA.W.GottaPH-adjusted bupivacaine and hyaluronidase for peribulbar blockAnesthesiology721990230232
- K.ZahlA.JordanJ.McGroartyB.SorensenA.W.GottaPeribulbar anesthesia. Effect of bicarbonate on mixtures of lidocaine, bupivacaine, and hyaluronidase with or without epinephrineOphthalmology981991239242
- G.ReahA.R.BodenhamP.BraithwaiteJ.EsmondM.J.MenagePeribulbar anesthesia using a mixture of local anesthetic and vecuroniumAnaesthesia531998551554
- Z.KucukyavuzM.K.AriciEffects of atracurium added to local anesthetics on akinesia in peribulbar blockReg. Anesth. Pain Med.272002487490
- D.MarhoferS.C.KettnerP.MarhoferS.PilsM.WeberM.ZeitlingerDexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer studyBr. J. Anaesth.11032013438442
- R.R.GandhiA.A.ShahI.PatelUse of dexmedetomidine along with bupivacaine for brachial plexus blockNatl. J. Med. Res.220126769
- S.HalderA.DasD.MandalM.ChandraS.RayM.R.BiswasP.MandalT.DasEffect of different doses of dexmedetomidine as adjuvant in bupivacaine-induced subarachnoid block for traumatized lower limb orthopedic surgery: a prospective, double-blinded and randomized controlled studyJ. Clin. Diagn. Res.81120140106
- A.M.AbdelhamidA.MahmoudM.M.AbdelhaqH.M.YasinA.BayoumiDexmedetomidine as an additive to local anesthetics compared with intravenous dexmedetomidine in peribulbar block for cataract surgerySaudi J. Anaesth.1020165054
- H.El-OzairyA.TharwatComparative study of the effect of adding two different doses of dexmedetomidine to levobupivacaine/hyaluronidase mixture on the peribulbar block in vitreoretinal surgeryAin-Shams J. Anesthesiol.072014393399
- R.A.FryJ.HendersonLocal anesthesia for eye surgery. The periocular techniqueAnaesthesia4519901417
- J.BarrN.KirkpatrickA.DickL.LeonardD.W.NobleEffects of adrenaline and hyaluronidase on plasma concentration of lignocaine and bupivacaine after peribulbar anaesthesiaBr. J. Anaesth.751995692697
- M.A.RamsayT.M.SavegeB.R.SimpsonR.GoodwinControlled sedation with alphaxalone-alphadoloneBMJ21974656659
- J.M.ChoiI.C.ChoiY.B.JeongT.H.KimK.D.HahmPretreatment of rocuronium reduces the frequency and severity of etomidate-induced myoclonusJ. Clin. Anesth.202008601604
- D.S.FriedmanS.W.ReevesE.B.BassL.H.LubomskiL.A.FleisherO.D.ScheinPatient preferences for anesthesia management during cataract surgeryBr. J. Ophthalmol.882004333335
- K.M.KempL.HenderlightM.NevillePrecedex: is it the future of cooperative sedation?Nursing38200878
- D.P.BhattacharjeeS.K.NayekS.DawnG.BandopadhyayK.GuptaEffects of dexmedetomidine on hemodynamic in patients undergoing laparoscopic cholecystectomy: a comparative studyJ. Anaesth. Clin. Pharmacol.2620104548
- J.C.EisenachM.De KockW.KlimschaAlpha (2)-adrenergic agonists for regional anesthesia. A clinical review of clonidineAnesthesiology851996655674
- T.Z.GuoJ.Y.JiangA.E.ButtermannM.MazeDexmedetomidine injection into the locus ceruleus produces antinociceptionAnesthesiology841996873881
- Y.AissaouilN.BelyamaniI.DrissikamilEffect of the addition of rocuronium to local anesthetics for peribulbar blockActa Anaesthesiol. Belg.6120105154
- A.A.AbdellatifM.A.El ShahawyA.I.AhmedW.A.AlmarakbiJ.A.AlhashemiEffects of local low-dose rocuronium on the quality of peribulbar anesthesia for cataract surgerySaudi J. Anaesth.52011360364
- T.Y.HamawyJ.N.BestarousRocuronium versus magnesium as an adjuvant to local anesthetics in peribulbar blockAin-Shams J. Anesthesiol.62013317321
- S.M.ChannabasappaV.R.ShettyS.K.DharmappaJ.SarmaEfficacy and safety of dexmedetomidine as an additive to local anesthetics in peribulbar block for cataract surgeryAnesth. Essays Res.720133943
- F.J.MacriS.J.CervarioClonidineArch. Ophthalmol.96197821112113
- J.VartianinenE.MacDonaldA.UrttiH.RonhiainenR.VirtanenDexmedetomidine induced ocular hypotension in rabbits with normal or elevated intraocular pressureInvest. Ophthalmol. Vis. Sci.33199220192023
- M.GeorgiouA.ParlapaniH.ArgiriadouP.PapagiannopoulouG.KatsikisE.KapriniSufentanil or clonidine for blunting the increase in intraocular pressure during rapid sequence inductionEur. J. Anaesthesiol.192002819822
- R.MadanN.Neerja BhartiD.ShendeS.K.KhokharH.L.KaulA dose response study of clonidine with local anesthetic mixture for peribulbar block: a comparison of three dosesAnesth. Analg.93200115931597